��Ŀ����
����Ŀ�����з�Ӧ�����ӷ���ʽ��ȷ���ǣ� ��
A.Al2O3����NaOH��Һ��Al2O3+2OH-=2AlO2-+2H2O
B.AgNO3��Һ�м��������ˮ��Ag++NH3��H2O=AgOH��+NH4+
C.�ö��Ե缫���0.1mol��L-1CuCl2��Һ��2Cl-+2H2O![]() H2��+Cl2��+2OH-
H2��+Cl2��+2OH-
D.����NaHCO3��Һ�ͳ���ʯ��ˮ��ϣ�Ca2++HCO![]() +OH-=CaCO3��+H2O
+OH-=CaCO3��+H2O
���𰸡�A
��������
A��Al2O3����NaOH��Һ����ƫ�����ƺ�ˮ��Al2O3+2OH-=2AlO2-+2H2O����A��ȷ��
B��AgNO3��Һ�м��������ˮ��������������������Һ��Ag++2NH3��H2O=Ag(NH3)2++2H2O ����B����
C���ö��Ե缫���0.1mol��L-1CuCl2��Һ����ͭ��������2Cl-+Cu2��![]() Cu+Cl2������C����
Cu+Cl2������C����
D������NaHCO3��Һ�ͳ���ʯ��ˮ��ϣ�Ca2��+2OH��+2HCO3��=CaCO3��+2H2O+CO32��,��D����
��ѡA��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���״���һ�ֿ�������Դ�����й����Ŀ�����Ӧ��ǰ����
��1������Pt/Al2O3��Pd/C��Rh/SiO2�����������������·�Ӧ���ϳɼ״���2H2(g)+CO(g)CH3OH(g) ��H<0
��������������Ǹ���ѧ���ļ��ܣ��÷�Ӧ����H=______���ú���ĸ�Ĵ���ʽ��ʾ����
��ѧ�� | H-H | C��O | C-H | C-O | O-H |
����/(kJ/mol) | a | b | c | d | e |
����һ�������£���1 molCO��2 molH2ͨ���ܱ������н��з�Ӧ�����ı�ijһ����������¶Ȼ�ѹǿ��ʱ��CH3OH�����������(CH3OH)�仯������ͼ��ʾ��

X����a�����ֵ��b��_________��������������С������ijͬѧ��Ϊ��ͼ��Y���ʾ�¶ȣ�����Ϊ���жϵ�������_______________��
��2����CO2��H2�ϳɼ״���3H2(g) + CO2(g)CH3OH(g) + H2O(g) ��H=��49.0kJ��mol��1����T��ʱ���ס��ҡ�������2L�ĺ����ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ƽ��ʱ�й��������£�
���� | �� | �� | �� | |
��ʼ��Ӧ��Ͷ���� | 3molH2(g) 1molCO2(g) | 1molCH3OH(g) 1molH2O(g) | 2molCH3OH(g) 2molH2O(g) | |
ƽ������ | c(CH3OH)/molL-1 | c1 | c2 | c3 |
��Ӧ�������仯/kJ | x | y | z | |
��ϵѹǿ/Pa | p1 | p2 | p3 | |
��Ӧ��ת���� | ��1 | ��2 | ��3 | |
�ټ�����20s�ﵽƽ��ʱ���x=29.4�������ƽ����Ӧ����v(CO2)=______��
������˵���������________������ĸ��ţ���
A��2c2<c3 B��z>2y C��p3<2p1 D����1+��3=1
��3���״���ˮ���������������⡣��֪��
�״��ֽⷴӦ��CH3OH(g)CO(g) + 2H2(g) ��H1=+90.64 kJ��mol��1
ˮ�����任��Ӧ��CO(g)+H2O(g)CO2(g)+H2(g) ��H2=��41.20 kJ��mol��1
�ٿ�ѧ��ͨ���ܶȷ��������о��״���ˮ�����������ⷴӦ����ʱ���õ��״���Pd(��)���淢������ʱ�ĸ�·�������������ϵ��ͼ��ʾ�����и���Pd(��)�����������*��ע���������л�����ķ�Ӧ����ʽΪ______________��
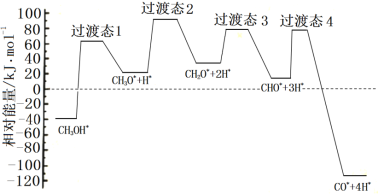
��573.2Kʱ����һ�����ܱ������г���5.00MPaCH3OHʹ��ֽ⣬th���ƽ��ʱ������ѹǿ��Ϊ10.00MPa����ƽ�ⳣ����ѹKp =_________��
����0.1MPa�£����ܽ�����1mol��n(CH3OH):n(H2 O)=1:1.3�Ļ���������һ�����ܱ������з�Ӧ��ʵ����ˮú���任��Ӧ���������¶ȵ����������½���ԭ����______________��