��Ŀ����
��8�֣�
(1) �����ͬ��pH��ͬ������ʹ�����Һ�ֱ��������Ŀ�����С��ͬ��п����Ӧ,��ʼʱ��������������_____________,��ַ�Ӧ������������_____________(��ͬ������Ķࡢ����Ķ�)
(2) ��һ������������Һ�м���������п��,��ʹ���������������ֲ��䣬����Ӧ���ʼӿ죬�ɼ���__________���壬Ҫʹ����������������,����Ӧ���ʼ���,�ɼ���____________���塣
��ѡ��ľ����У�
(A) ���� (B)�ռ� (C)���� (D) ������ (E) KHSO4
(3)ij�о�С�����ö����ķ�������Al��Fe�ֱ����ᷴӦ�Ŀ��������������ͼ1��ʾ��װ�á�
�� �����߿������Ӻ��ʵ�װ��___________________
����Ҫ�Ƚϲ�������Ŀ��������Բ�����ͬʱ����ڲ�������������Ҳ���Բ���____________________________________��
��ʵ������˿�������������(v)��ʱ��(t)�Ĺ�ϵ��ͼ2��ʾ����t1��t2ʱ����ڷ�Ӧ�����ӿ����Ҫԭ����__________________________________��
��8�֣�
��1��һ���������
��2��C��D
(3)��B �ڲ�����ͬ�������������ʱ�䡡�۷�Ӧ���ȣ���Һ�¶�����
����:

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�A��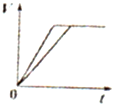 ��ʾ���ʵ���֮��Ϊ2��3��þ�����ֱ������ϡ���ᷴӦʱ����������������V����ʱ�䣨t���Ĺ�ϵ | B��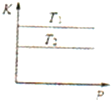 ��ʾ2SO2��g��+O2��g��?2SO3��g������H��0����ƽ�ⳣ��K��ѹǿP�Ĺ�ϵ��T1��T2 | C�� ��ʾ��0.1mol-L-1NaOH��Һ�ֱ�ζ�Ũ�Ⱦ�Ϊ0.1mol?L-1�����ͬ������ʹ��ᣬ���е������ǵζ���������� | D��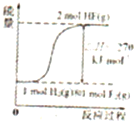 ��ʾ����ͬ������1 mol H2��g����1 mol F2��g����Ӧ����2 mol HF��g��ʱ���ų�����ͯΪ270kJ |
 2NH3��g����H=-92kJ?mol-1
2NH3��g����H=-92kJ?mol-1 Ba2+��aq��+SO42-��aq��
Ba2+��aq��+SO42-��aq�� H++HCO3-
H++HCO3-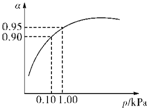 ����������Ҫ����Ƿ��������ȣ��������Ʒ����������������裮
����������Ҫ����Ƿ��������ȣ��������Ʒ����������������裮 3C��g������֪����1molA��3molB��ƽ��ʱA�����ʵ���Ϊa mol��
3C��g������֪����1molA��3molB��ƽ��ʱA�����ʵ���Ϊa mol��