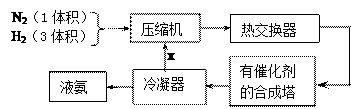��Ŀ����
�Ӻ�ͭ��������Ͳ��Ŀ�״������������ȡ����������һ�ֹ������£�

�������Ϲ��ջش��������⣺
��1�����ʱ����_____________Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ���������ĵ缫��Ӧ����ʽΪ
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ�������ӣ��ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��3��д������ܵ����ӷ���ʽ��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Au +6HNO3(Ũ)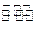 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
��5��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ
____________________________________________________________________________

�������Ϲ��ջش��������⣺
��1�����ʱ����_____________Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ���������ĵ缫��Ӧ����ʽΪ
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ�������ӣ��ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��3��д������ܵ����ӷ���ʽ��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Au +6HNO3(Ũ)
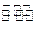 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮�� ��5��д����Ӧ�ݵĻ�ѧ��Ӧ����ʽ
____________________________________________________________________________
����15�֣�
��1���������ϣ�1�֣���Cu2+ + 2e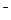 �� Cu��2�֣�
�� Cu��2�֣�
��2��2Ag(NH3)2+ + 2OH��+CH2OH(CHOH)4CHO��CH2OH(CHOH)4COO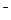 + NH4+ + 2Ag��+ 3NH3 + H2O��ˮԡ���ȣ���3�֣�
+ NH4+ + 2Ag��+ 3NH3 + H2O��ˮԡ���ȣ���3�֣�
��3��2AuCl4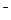 + 3SO2 + 6 H2O =" 2Au" + 8Cl
+ 3SO2 + 6 H2O =" 2Au" + 8Cl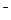 + 3SO42
+ 3SO42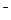 + 12H+��3�֣�
+ 12H+��3�֣�
��4����ˮ�к��д�����Cl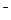 ��Au3+��Cl��������AuCl4
��Au3+��Cl��������AuCl4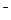 ��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ�����ƣ���������ˮ����3�֣�
��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ�����ƣ���������ˮ����3�֣�
��5��3(NH4)2 PtCl6 ="=" 3Pt + 2N2 +18HCl + 2NH3��435�棩��3�֣�
��1���������ϣ�1�֣���Cu2+ + 2e
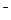 �� Cu��2�֣�
�� Cu��2�֣���2��2Ag(NH3)2+ + 2OH��+CH2OH(CHOH)4CHO��CH2OH(CHOH)4COO
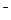 + NH4+ + 2Ag��+ 3NH3 + H2O��ˮԡ���ȣ���3�֣�
+ NH4+ + 2Ag��+ 3NH3 + H2O��ˮԡ���ȣ���3�֣���3��2AuCl4
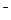 + 3SO2 + 6 H2O =" 2Au" + 8Cl
+ 3SO2 + 6 H2O =" 2Au" + 8Cl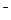 + 3SO42
+ 3SO42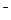 + 12H+��3�֣�
+ 12H+��3�֣���4����ˮ�к��д�����Cl
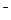 ��Au3+��Cl��������AuCl4
��Au3+��Cl��������AuCl4 ��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ�����ƣ���������ˮ����3�֣�
��ʹ��ƽ����Au3+Ũ�Ƚ��ͣ�ƽ�����ƣ���������ˮ����3�֣���5��3(NH4)2 PtCl6 ="=" 3Pt + 2N2 +18HCl + 2NH3��435�棩��3�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 -﮻�ʯ����Ҫ�ɷ�ΪLi2O
-﮻�ʯ����Ҫ�ɷ�ΪLi2O Al2O3
Al2O3 ��Li2CO3�Ĺ����������£�
��Li2CO3�Ĺ����������£�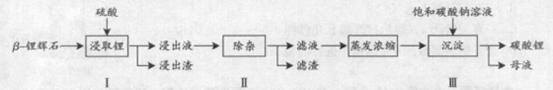


 ��
�� ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� ��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� �� 2NH3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK��
2NH3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK��
 ����Y�Ľṹ��ʽΪ������������������������
����Y�Ľṹ��ʽΪ������������������������
 Si��s����4HCl(g)��
Si��s����4HCl(g)��
 ʽ)��
ʽ)��