��Ŀ����
��8�֣�����ѧ����ѧ�뼼����
���Ṥҵ����Ӧ�����ۺϾ���Ч�����⡣
��1�����������ĸ�������ѡ��һ���½�һ�����᳧������Ϊ��ַ��ѡ�� �Ľ��������ţ���
A���зḻ��������Դ�ij��� B��������������γ���
C��������������Ĺ�ҵ���� D���˿ڳ��ܵ��Ļ�����ҵ���ij���
��2��CuFeS2 �ǻ��������һ�ɷ֣�����ʱCuFeS2 ת��ΪCuO��Fe2O3��SO2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�������Ṥҵ�Ʒ��У���������������˵��������������Ҫԭ����߶�����ȷ���� ��
A��������ȼ��ǰ��Ҫ���飬��Ϊ���Ļ��������ڿ�����ȼ��
B���ӷ���¯������¯���辻������Ϊ¯���ж�������������ʷ�Ӧ
C��������������Ϊ��������ʱ��ʹ�ô���������������߶��������ת����
D������������98.3%��Ũ�������գ�Ŀ���Ƿ�ֹ�γ�������������������������Ч��
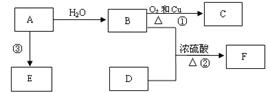
��4�������᳧����¯�ų��Ŀ����к���Fe2O3��CuO��CuSO4����CuO��SO3�ڷ���¯�л��϶��ɣ�����������ͭ���������������¯�¶Ȳ�ͬ���仯�����±���
��֪CuSO4�ڵ���660��ʱ����ֽ⣬���Ҫ�����ϱ���CuSO4 �������������¶����߶����͵�ԭ�� ��
���Ṥҵ����Ӧ�����ۺϾ���Ч�����⡣
��1�����������ĸ�������ѡ��һ���½�һ�����᳧������Ϊ��ַ��ѡ�� �Ľ��������ţ���
A���зḻ��������Դ�ij��� B��������������γ���
C��������������Ĺ�ҵ���� D���˿ڳ��ܵ��Ļ�����ҵ���ij���
��2��CuFeS2 �ǻ��������һ�ɷ֣�����ʱCuFeS2 ת��ΪCuO��Fe2O3��SO2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�������Ṥҵ�Ʒ��У���������������˵��������������Ҫԭ����߶�����ȷ���� ��
A��������ȼ��ǰ��Ҫ���飬��Ϊ���Ļ��������ڿ�����ȼ��
B���ӷ���¯������¯���辻������Ϊ¯���ж�������������ʷ�Ӧ
C��������������Ϊ��������ʱ��ʹ�ô���������������߶��������ת����
D������������98.3%��Ũ�������գ�Ŀ���Ƿ�ֹ�γ�������������������������Ч��
��4�������᳧����¯�ų��Ŀ����к���Fe2O3��CuO��CuSO4����CuO��SO3�ڷ���¯�л��϶��ɣ�����������ͭ���������������¯�¶Ȳ�ͬ���仯�����±���
| ����¯�¶�/�� | 600 | 620 | 640 | 660 |
| ¯����CuSO4����������/% | 9.3 | 9.2 | 9.0 | 8.4 |
��1��C��2�֣� ��2��4CuFeS2+13O2 4CuO+2Fe2O3+8SO2��2�֣� ��3��D��2�֣�
4CuO+2Fe2O3+8SO2��2�֣� ��3��D��2�֣�
��4��SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ��1�֣������¶����ߣ�ƽ�����ƣ�SO3���ʵ������٣�1�֣�������CuSO4�������٣����¶����ߣ�SO3���ʵ������٣���CuSO4�������٣�
 4CuO+2Fe2O3+8SO2��2�֣� ��3��D��2�֣�
4CuO+2Fe2O3+8SO2��2�֣� ��3��D��2�֣���4��SO2ת��ΪSO3������Ӧ���ȵĿ��淴Ӧ��1�֣������¶����ߣ�ƽ�����ƣ�SO3���ʵ������٣�1�֣�������CuSO4�������٣����¶����ߣ�SO3���ʵ������٣���CuSO4�������٣�
��

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ


 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮�� 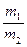 =x(m1��m2�ֱ�Ϊ50%��HNO3��������90%��HNO3������)������17tҺ��Ϊԭ������HNO3��
=x(m1��m2�ֱ�Ϊ50%��HNO3��������90%��HNO3������)������17tҺ��Ϊԭ������HNO3�� 2NH3(g) ���ڷ�Ӧ�����У�
2NH3(g) ���ڷ�Ӧ�����У� t1��t2��t3��t4ʱ�����ı䣬����Ӧ���ʷ����仯������ͼ�����ڿ��ܵ������ı������ж���ȷ����
t1��t2��t3��t4ʱ�����ı䣬����Ӧ���ʷ����仯������ͼ�����ڿ��ܵ������ı������ж���ȷ����
 ( )
( )