��Ŀ����
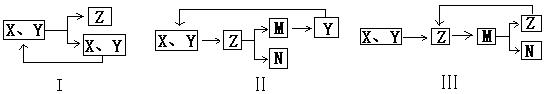
��10�֣���ͼ�ǹ�ҵ������������̡�

�ϳ�������������ý������¯������Pt��Rh�Ͻ�������ش��������⣺
�� 1909�껯ѧ�ҹ�����ʵ�����״κϳ��˰���2007�껯ѧ�Ҹ���¡����ض��ڹ����о���֤ʵ�������뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ�����£�

 ��
�� ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� ��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� ��
�� �ϳɰ���Ӧ�Ļ�ѧ����ʽΪN2(g)��3H2(g) 2NH3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK��
2NH3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK��
����һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1(�����)��Ϻ����ϳ�������Ӧ�ﵽƽ��ʱ��ƽ��������NH3���������Ϊ15������ʱH2��ת����Ϊ ��
�� ��֪��4NH3(g)��3O2(g)��2N2(g)��6H2O(g) ��H����1266.8 kJ/mol
N2(g)��O2(g)��2NO(g) ��H����180.5 kJ/mol
�����������Ȼ�ѧ����ʽΪ ��
�� ��������ͨ�������Ŀ���� ��

�ϳ�������������ý������¯������Pt��Rh�Ͻ�������ش��������⣺
�� 1909�껯ѧ�ҹ�����ʵ�����״κϳ��˰���2007�껯ѧ�Ҹ���¡����ض��ڹ����о���֤ʵ�������뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ�����£�

 ��
�� ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� ��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� ���� �ϳɰ���Ӧ�Ļ�ѧ����ʽΪN2(g)��3H2(g)
 2NH3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK��
2NH3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK������һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1(�����)��Ϻ����ϳ�������Ӧ�ﵽƽ��ʱ��ƽ��������NH3���������Ϊ15������ʱH2��ת����Ϊ ��
�� ��֪��4NH3(g)��3O2(g)��2N2(g)��6H2O(g) ��H����1266.8 kJ/mol
N2(g)��O2(g)��2NO(g) ��H����180.5 kJ/mol
�����������Ȼ�ѧ����ʽΪ ��
�� ��������ͨ�������Ŀ���� ��
�� ͼ�ڱ�ʾN2��H2�������ڴ������棬ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ������
�� K��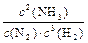 26��
26��
�� 4NH3(g)��5O2(g)��4NO(g)��6H2O(g) ��H����905.8kJ/mol
�� ʹNOѭ�����ã�ȫ��ת����HNO3
�� K��
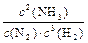 26��
26���� 4NH3(g)��5O2(g)��4NO(g)��6H2O(g) ��H����905.8kJ/mol
�� ʹNOѭ�����ã�ȫ��ת����HNO3
�����Թ�ҵ����HNO3�ı��������黯ѧ����֪ʶ���漰��ѧƽ�ⳣ����ת���ʵļ��㣬��˹����Ӧ�õȣ����е��⡣��2����N2��H2�����ʵ�����Ϊa,3a mol��N2��ת����Ϊx��
N2(g)��3H2(g) 2NH3(g)
2NH3(g)
��ʼ a 3a 0
ƽ�� a-x 3a-3x 2x
ת�� x 3x 2x
NH3%=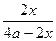 =15%��H2��ת����Ϊ��
=15%��H2��ת����Ϊ��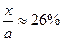 ��
��
��3���ܷ�Ӧ���ɢ�+�ڡ�2��ã�?H��������һ��������㡣
��4����ҵ����HNO3Ϊѭ����Ӧ��Ϊ��ʹNԪ����ʧ���ʿ���ͨ��O2���䷴Ӧ��
N2(g)��3H2(g)
 2NH3(g)
2NH3(g)��ʼ a 3a 0
ƽ�� a-x 3a-3x 2x
ת�� x 3x 2x
NH3%=
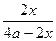 =15%��H2��ת����Ϊ��
=15%��H2��ת����Ϊ��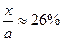 ��
����3���ܷ�Ӧ���ɢ�+�ڡ�2��ã�?H��������һ��������㡣
��4����ҵ����HNO3Ϊѭ����Ӧ��Ϊ��ʹNԪ����ʧ���ʿ���ͨ��O2���䷴Ӧ��

��ϰ��ϵ�д�
�����Ŀ

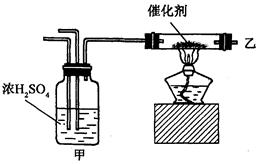
 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮�� 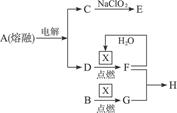
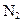 ��
��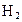 ��
��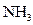
 ( )
( )