��Ŀ����
��16�֣�̼��﮹㷺Ӧ�����մɺ�ҽҩ�������� -﮻�ʯ����Ҫ�ɷ�ΪLi2O
-﮻�ʯ����Ҫ�ɷ�ΪLi2O Al2O3
Al2O3 4SiO2��Ϊԭ������
4SiO2��Ϊԭ������ ��Li2CO3�Ĺ����������£�
��Li2CO3�Ĺ����������£�
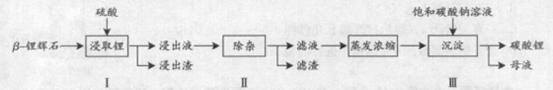
��֪��Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��9.7��12.4��Li2SO4��LiOH��Li2CO3��303K�µ��ܽ�ȷֱ�Ϊ34.2g��12.7g��1.3g��
(1)�����ǰ��B-﮻�ʯҪ�����ϸ������Ŀ����_____________��
(2)������У������õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼���_____________(�ʯ��ʯ�������Ȼ��ơ���ϡ���ᡱ)�Ե�����Һ��pH��6.0~6.5�����������������ӣ�Ȼ�����õ�����Һ��
(3)����II�У���������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У��ɳ�ȥ���Ӽ�����������
_________________��
��4������III�У����ɳ��������ӷ���ʽΪ________________��
��5����ĸҺ�пɻ��յ���Ҫ������__________________��
 -﮻�ʯ����Ҫ�ɷ�ΪLi2O
-﮻�ʯ����Ҫ�ɷ�ΪLi2O Al2O3
Al2O3 4SiO2��Ϊԭ������
4SiO2��Ϊԭ������ ��Li2CO3�Ĺ����������£�
��Li2CO3�Ĺ����������£�
��֪��Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��5.2��9.7��12.4��Li2SO4��LiOH��Li2CO3��303K�µ��ܽ�ȷֱ�Ϊ34.2g��12.7g��1.3g��
(1)�����ǰ��B-﮻�ʯҪ�����ϸ������Ŀ����_____________��
(2)������У������õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+�����ʣ����ڽ����¼���_____________(�ʯ��ʯ�������Ȼ��ơ���ϡ���ᡱ)�Ե�����Һ��pH��6.0~6.5�����������������ӣ�Ȼ�����õ�����Һ��
(3)����II�У���������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ�У��ɳ�ȥ���Ӽ�����������
_________________��
��4������III�У����ɳ��������ӷ���ʽΪ________________��
��5����ĸҺ�пɻ��յ���Ҫ������__________________��
��1���ӿ췴Ӧ����
��2��ʯ��ʯ
��3��Fe2+��Mg2+��Ca2+
(4)2Li+ + CO32- =Li2CO3��
��5��NaOH
��

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� ������ϵ�д�
������ϵ�д�
�����Ŀ
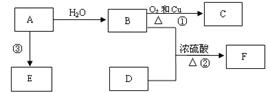

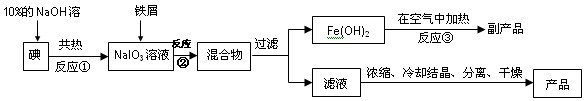

 Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��
Au(NO3)3+3NO2��+ 3H2O����÷�Ӧ������еij̶ȼ�С�����Խ�������Ũ���ᣬ����ȴ����������ˮ���Լ�Ҫ����֮��  2NH3(g) ���ڷ�Ӧ�����У�
2NH3(g) ���ڷ�Ӧ�����У� t1��t2��t3��t4ʱ�����ı䣬����Ӧ���ʷ����仯������ͼ�����ڿ��ܵ������ı������ж���ȷ����
t1��t2��t3��t4ʱ�����ı䣬����Ӧ���ʷ����仯������ͼ�����ڿ��ܵ������ı������ж���ȷ����