��Ŀ����
3��ijX��Һ��ʹ���ȳʺ�ɫ���ڸ���Һ�п��ܺ���K+��Fe2+��A13+��NH4+��CO32-��SO32-��SO42-��AlO2-��SiO32-��C1-�е������֣���ȡX��Һ��������ʵ�飬ʵ��������������£�����˵����ȷ���ǣ�������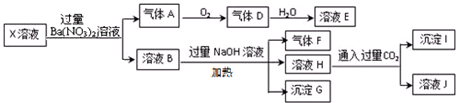
| A�� | X�п϶�����Fe2+��A13+��NH4+��C1- | |
| B�� | X�в���ȷ���������� K+��C1- | |
| C�� | ����G�ڿ����з��ù����У�������ɫ���ɰ�ɫ��Ϊ����ɫ����Ϊ���ɫ | |
| D�� | ��״���½�һ�Թ�D���嵹����ˮ���г�����գ�����������Һû����ɢ����������Һ���ʵ���Ũ��ԼΪ0.036mol/L |
���� ǿ������Һ��һ��û��CO32-��SO32-��AlO2-��SiO32-���������ᱵ���������������Һ����ǿ�����ԣ�һ�������������ӣ���A��һ��������D�Ƕ���������E���������C�����ᱵ����Һ��һ���������������������������ƣ�����������F�ǰ�����ԭ��Һ��һ������笠����ӣ������˳���G��ΪFe��OH��3��H��ͨ�������̼�����ɳ���I������Ϊ�����������������Ϸ�������ѡ����һ�жϣ�
��� �⣺ijX��Һ��ʹ���ȳʺ�ɫ����X��ǿ������Һ����ԭ��Һ��һ��û��CO32-��SO32-��AlO2-��SiO32-��
�������ᱵ����Һ������ǿ�����ԣ��ܲ�������A����ԭ��Һ��һ����Fe2+��Fe2+��NO3-�����������·���������ԭ��Ӧ���ɵ�����A��NO��D��NO2��E��ΪHNO3�������������ɣ���ԭ��Һ����SO42-��������ҺҪ�ʵ����Կ�֪����Һ��һ����C1-������Һ��һ�����л�ԭ�Ե�����Fe2+��C1-��һ������SO42-��
B�м����������Ʋ���������F��Fһ���ǰ�������Һ��һ������NH4+������Fe2+������ΪFe3+�������ɵij���GΪFe��OH��3��
H��ͨ������Ķ�����̼�����˳���I����IΪ��������������Һ��һ������A13+��
����Һ��һ����Fe2+��A13+��NH4+��C1-��һ������CO32-��SO32-��AlO2-��SiO32-��SO42-��K+�Ĵ��ڲ���ȷ����
A����Һ��һ����Fe2+��A13+��NH4+��C1-����A��ȷ��
B�����ݷ�����C1-һ�����ڣ���B����
C������Fe2+������ΪFe3+�������ɵij���GΪFe��OH��3�����ᷢ������ɫ�仯����C����
D��������������ݻ�Ϊ3L����������������Ϊ3L������������ˮ��Ӧ�ķ���ʽΪ��
3NO2 +H2O=2HNO3+NO��
3 2
$\frac{3}{22.4}$mol $\frac{2}{22.4L}$
��Ӧǰ������������3L��Ϊ1L��������Һ�����Ϊ2L������Һ������Ϊ���ᣬ
������������ʵ���Ũ��ΪC=$\frac{n}{V}$=$\frac{\frac{2}{22.4}mol}{2L}$=0.045mol/L��
��D����
��ѡA��
���� ���⿼���˶������������ʼ����ʵ���Ũ�ȵļ��㣬��Ϊ����������ˮ��Ӧ�����������ɣ�������Һ���ܳ�����������������Һ�����ֻռ�����ݻ���$\frac{2}{3}$��������ȷ�ж���Һ������ǽⱾ��Ĺؼ���

| A�� | �ƹ�ʹ�þ۶�����̼�ɽ������ϣ��ܼ��ٰ�ɫ��Ⱦ | |
| B�� | ͨ����˵�������л��ϳɲ�����ָ���ϡ��ϳ���ά���ϳ��� | |
| C�� | ʵ�顰ú��������ú�ĵ硱�����ȼ�ϸ��칤�̣������ڱ������� | |
| D�� | �����������о���������������Ϊ�����в����������Ķ������� |
 ��1��һ�������£���һ���������ܱ������г���2mol SO2��1mol O2������Ӧ��2SO2��g��+O2��g���T2SO3��g��������˵���У���ȷ����C��
��1��һ�������£���һ���������ܱ������г���2mol SO2��1mol O2������Ӧ��2SO2��g��+O2��g���T2SO3��g��������˵���У���ȷ����C��A����������O2��=2���棨SO3����˵���ÿ��淴Ӧ�Ѵﵽƽ��״̬
B�������¶Ⱥ�����������䣬����2mol N2����ѧ��Ӧ���ʼӿ�
C��ƽ����ƶ�����ѹ�����壬�ﵽ��ƽ��ʱSO2��O2�İٷֺ�����С��SO3�İٷֺ�������
D����ƽ������¶Ⱥ�����������䣬�ٳ���2mol SO2����ѧƽ�������ƶ���SO2��ת��������
��2��β��SO2��NaOH��Һ���պ������Na2SO3����ʵ��ⶨ������0.1mol/L Na2SO3��Һ��pHΪ8������Һ����ˮ����������������ӵ�Ũ��Ϊ10-6��
��3��ú̿�е�����Ҫ�Ի�������ʽ���ڣ��������ѳ��������������ط�Ӧ��������������ط�Ӧ��ƽ�ⳣ���Ķ���ֵ���¶ȵĹ�ϵ���£�
| ��ط�Ӧ | ��Ӧ�� | ƽ�ⳣ��K |
| FeS2��s��+H2��g��?FeS��s��+H2S��g�� | ��H1 | K1 |
| $\frac{1}{2}$FeS2��s��+H2��g��?$\frac{1}{2}$Fe��s��+H2S��g�� | ��H2 | K2 |
| FeS��s��+H2��g��?Fe��s��+H2S��g�� | ��H3 | K3 |
���������ѳ��ʿɲ�ȡ�Ĵ�ʩ�������¶ȣ���1������
��1000Kʱ��ƽ�ⳣ���Ķ���lgK1=lgK2-lgK3����lgK2��lgK3����ʾ����
���� ������Ϣ��
������˵������ȷ���ǣ�������
| A�� | Xֻ��1�ֽṹ | |
| B�� | ������Ϣ�ؿ�����ϩ������ʹ��ˮ��ɫ | |
| C�� | ����������ӦʽΪ��C21H41COOH+X+2H2O-2e-��C23H46+2CO32-+6H+ | |
| D�� | �����Ļ�ԭ����ΪH2 |
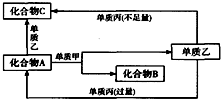 A��B��C����������Ԫ����ɵ�������Ǻ͵��ʼס��ҡ�������ͼ��ʾ��ת����ϵ��ת�����̶�����Ҫʹ�ô�������
A��B��C����������Ԫ����ɵ�������Ǻ͵��ʼס��ҡ�������ͼ��ʾ��ת����ϵ��ת�����̶�����Ҫʹ�ô�������
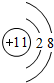 ���Ļ�ѧʽNa2S2O4
���Ļ�ѧʽNa2S2O4 �������������Ħ���Σ���ѧʽΪx��NH4��2SO4•yFeSO4•zH2O����������ˮ������ȱ����ƶѪ�ȣ�ijʵ��С����������ķ������ⶨ����ɣ�
�������������Ħ���Σ���ѧʽΪx��NH4��2SO4•yFeSO4•zH2O����������ˮ������ȱ����ƶѪ�ȣ�ijʵ��С����������ķ������ⶨ����ɣ�