��Ŀ����
16������˵����ȷ���ǣ�������| A�� | ��֪30��ʱKw=3.80��10-14����30��ʱc��H+��=1.00��10-7mol/L����Һ�����Ե� | |
| B�� | ��������θҺ��pH=1.4��Ӥ���������������ͬ��θҺ��pH=5.0�������˵��������²���25�棬������θҺ��c��OH-�����������103.6�� | |
| C�� | ������ˮ�м���������NaHSO3���岻��ʹc��HClO������ | |
| D�� | ������״̬�£�1molKHSO4��ȫ���������������ĿΪ2NA |
���� A��30��ʱKw=3.80��10-14����30��ʱc��H+��=1.00��10-7mol/L����Һ��c��OH-��=3.80��10-7mol/L������Һ�������ȡ����c��OH-����c��H+������Դ�С��
B�������˵����µ��¶��£�ˮ�����ӻ�Kw=x���ݴ˱�ʾ������θҺ��Ӥ��θҺ�е�c��OH-�����ݴ˷�����
C��NaHSO3������ǿ��ԭ�ԣ��ܽ�HClO��ԭ��
D��KHSO4������״̬��ֻ�ܵ���Ϊ�����Ӻ�����������ӣ�
��� �⣺A��30��ʱKw=3.80��10-14����30��ʱc��H+��=1.00��10-7mol/L����Һ��c��OH-��=3.80��10-7mol/L����ʱc��OH-����c��H+��������Һ�Լ��ԣ���A����
B�������˵����µ��¶��£�ˮ�����ӻ�Kw=10-x������θҺ��pH=1.4����c��H+��=10-1.4mol/L��c��OH-��=101.4-xmol/L��Ӥ��θҺ��pH=5����c��H+��=10-5mol/L��c��OH-��=105-xmol/L������θҺ��c��OH-���ı�ֵΪ��$\frac{1{0}^{1.4-x}}{1{0}^{5-x}}$=10-3.6����������10-3.6������B����
C��NaHSO3������ǿ��ԭ�ԣ��ܽ�HClO��ԭΪCl-���ʼ���NaHSO3�����HClO��Ũ�ȼ�С����C��ȷ��
D��KHSO4������״̬��ֻ�ܵ���Ϊ�����Ӻ�����������ӣ���������״̬�£�1molKHSO4����ֻ�ܵ����1mol�����Ӽ�NA������D����
��ѡC��
���� ���⿼������Һ����Ե��жϡ�pH���йؼ��������֮��ķ�Ӧ���µ�ƽ����ƶ������⣬�ۺ��ԱȽ�ǿ���Ѷ����У�

| A�� | �����һ���µ�ͬ�������� | B�� | �����һ���µ�ͬλ�� | ||
| C�� | ������ɿ���H${\;}_{3}^{+}$��ʾ | D�� | ���Ļ�ѧ�������������� |
| A�� | CH4��C2H4ȼ������ˮ��������ͬ������������ͬ | |
| B�� | C2H4ȼ������ˮ��CO2�����ʵ������ | |
| C�� | CH4��������ߣ�ȼ������ˮ��� | |
| D�� | C2H2��̼����ߣ�ȼ�����ɵ�CO2��� |
| A�� | ���ڱ��д������£�����Ԫ���γɵĵ����۷е���� | |
| B�� | ͬ����Ԫ��ԭ�Ӱ뾶�����Ӱ뾶�ݱ������ͬ | |
| C�� | HF��HCl��HBr��HI���۷е������� | |
| D�� | �������Ӽ��Ļ�����һ�������ӻ����� |
 ��
�� ��
�� B��
B��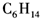 C��
C��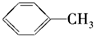 D��
D��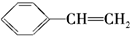
 ���÷���Ҳ������ij���ϵĵ��壬д���ϳɸ����ϵĻ�ѧ����ʽ��
���÷���Ҳ������ij���ϵĵ��壬д���ϳɸ����ϵĻ�ѧ����ʽ�� ��
��