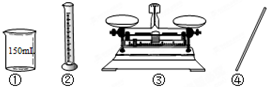
��Ŀ����
��18.4mol/L��Ũ����ϡ�ͳ�0.92mol/L��ϡ����100ml���ش��������⣺
��1��
��2�����Ʋ����ɷֽ�����¼�����
A������ƿ��ע����������ˮ���������ƿ�Ƿ�©ˮ
B����������ˮϴ���ձ�������Һע������ƿ�����ظ���������
C������ȴ������ע������ƿ��
D���ݼ��㣬����Ͳ��ȡһ�������Ũ����
E��Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F��������ƿ���ӣ���ҡ�ȣ�װƿ
G�ý�ͷ�ιܼ���������ˮ��ʹ��Һ����ǡ����̶�����
H����������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���
��ȷ�IJ���˳���ǣ�A______F
��3�����в��������ʹ��Һ���ʵ���Ũ��ƫ�͵���______
Aû�н�ϴ��Һת�Ƶ�����ƿ��
B����ƿϴ����δ�����ﴦ��
Cת�ƹ���������������Һ����
Dҡ�Ⱥ������۲죬������Һδ��̶��ߣ�û�����õιܼӼ�������ˮ���̶��ߣ�
��1��
| ӦȡŨ��������/ml | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
A������ƿ��ע����������ˮ���������ƿ�Ƿ�©ˮ
B����������ˮϴ���ձ�������Һע������ƿ�����ظ���������
C������ȴ������ע������ƿ��
D���ݼ��㣬����Ͳ��ȡһ�������Ũ����
E��Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F��������ƿ���ӣ���ҡ�ȣ�װƿ
G�ý�ͷ�ιܼ���������ˮ��ʹ��Һ����ǡ����̶�����
H����������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���
��ȷ�IJ���˳���ǣ�A______F
��3�����в��������ʹ��Һ���ʵ���Ũ��ƫ�͵���______
Aû�н�ϴ��Һת�Ƶ�����ƿ��
B����ƿϴ����δ�����ﴦ��
Cת�ƹ���������������Һ����
Dҡ�Ⱥ������۲죬������Һδ��̶��ߣ�û�����õιܼӼ�������ˮ���̶��ߣ�
��1������100mL 0.92mol/L��ϡ���ᣬ��Ҫѡ��100mL����ƿ�����Ƶ�ϡ�����к�����������ʵ���Ϊ��0.92mol/L��0.1L=0.092mol����Ҫ18.4mol/L��Ũ��������Ϊ��
=0.005L=5.0mL�����ƹ�������Ҫʹ�õ���������100mL����ƿ�⣬����Ҫ�������У���Ͳ���ձ�������������ͷ�ιܣ�
�ʴ�Ϊ��5.0��100����Ͳ���ձ�������������ͷ�ιܣ�
��2������100mL 0.92mol/L��ϡ����IJ���Ϊ����©�����㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ����˳��Ϊ��ADECBHGF��
�ʴ�Ϊ��DECBHG��
��3��Aû�н�ϴ��Һת�Ƶ�����ƿ�У��������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���A��ȷ��
B����ƿϴ����δ�����ﴦ��������Һ��������ʵ����ʵ�����û��Ӱ�죬���Բ�Ӱ�����ƽ������B����
Cת�ƹ���������������Һ�������������Ƶ���Һ�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���C��ȷ��
Dҡ�Ⱥ������۲죬������Һδ��̶��ߣ�û�����õιܼӼ�������ˮ���̶��ߣ��ò�����ȷ����������ƽ������Ӱ�죬��D����
��ѡAC��
| 0.092mol |
| 18.4mol/L |
�ʴ�Ϊ��5.0��100����Ͳ���ձ�������������ͷ�ιܣ�
��2������100mL 0.92mol/L��ϡ����IJ���Ϊ����©�����㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ����˳��Ϊ��ADECBHGF��
�ʴ�Ϊ��DECBHG��
��3��Aû�н�ϴ��Һת�Ƶ�����ƿ�У��������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���A��ȷ��
B����ƿϴ����δ�����ﴦ��������Һ��������ʵ����ʵ�����û��Ӱ�죬���Բ�Ӱ�����ƽ������B����
Cת�ƹ���������������Һ�������������Ƶ���Һ�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���C��ȷ��
Dҡ�Ⱥ������۲죬������Һδ��̶��ߣ�û�����õιܼӼ�������ˮ���̶��ߣ��ò�����ȷ����������ƽ������Ӱ�죬��D����
��ѡAC��

��ϰ��ϵ�д�
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
�����Ŀ