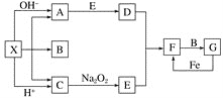
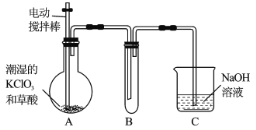
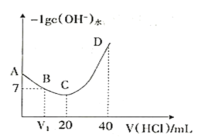
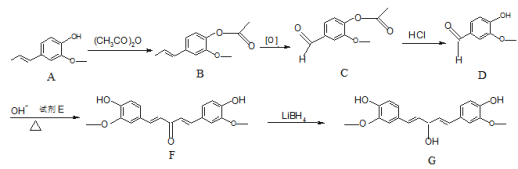
��Ŀ����
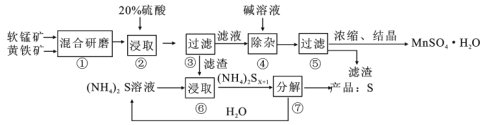
����Ŀ��һ�������̿�(��Ҫ�ɷ�MnO2)�ͻ�����(��Ҫ�ɷ�FeS2)��ȡMnSO4��H2O�����յ�����Ĺ����������£�
(1)����ٻ����ĥ��ϸ�۵���ҪĿ����__________������ڽ�ȡʱ������S��MnSO4��Fe2(SO4)3����Ӧ�����ӷ���ʽΪ___________��
(2)���������������Һ���ܺ���Fe2+������Fe2���ķ�����________����ȥFe2+�ķ�����__________����������ƻ�Fe(OH)3���岢ʹ�������������ڹ��˷��롣�������������Ϊ___________(�ѧʽ)��
(3)���������90��100���½��У��÷�Ӧ�Ļ�ѧ����ʽΪ___________��
(4)���ˮ������ӷ���ʽΪ��S2-��![]() ��H2O
��H2O![]() HS-��NH3��H2O������ж���Һ�������______��
HS-��NH3��H2O������ж���Һ�������______��
(5)�ⶨ��ƷMnSO4��H2O�ķ���֮һ�ǣ�ȷ��ȡa g��Ʒ����ƿ�У���������ZnO��H2O��У�Ȼ����c mol��L-1 KMnO4����Һ�ζ���dz��ɫ�Ұ���Ӳ��ʣ����ı���ҺV mL���ζ���Ӧ�����ӷ���ʽΪ2![]() ��3Mn2+��2H2O��5MnO2����4H+����Ʒ��Mn2+����������Ϊ��(Mn2+)��___________��
��3Mn2+��2H2O��5MnO2����4H+����Ʒ��Mn2+����������Ϊ��(Mn2+)��___________��
���𰸡���������ȡʱ�Ľ�ȡ�� 3MnO2 +2FeS2+12H+=3Mn2++2Fe3++4S��+6H2O ȡ������Һ���Թ��У�������������Һ����ɫ��ȥ ����H2O2��MnO2 Fe(OH)3 (NH4)2Sx+1=2NH3��+H2S��+xS�� �Ƚ�SH-��![]() ˮ�ⳣ������Դ�С
ˮ�ⳣ������Դ�С ![]() ��100��
��100��
��������
���̿��̿����ĥ��������Ӵ�������ӿ췴Ӧ���ʣ���������ܽ��̣�������轫��Һ��������Ȼ���ڲ��Ͻ����¼���Ŀ���ƻ������������壬ʹ֮�������õ���������Һ����Ũ�����ᾧ�õ�MnSO4��H2O���ݴ˽��
(1)����ٻ����ĥ��ϸ�ۣ���������Ӧ��ĽӴ��������������ȡʱ�Ľ�ȡ�ʣ������ȡ�ķ�Ӧ����ʽΪ��3MnO2 +2FeS2+12H+=3Mn2++2Fe3++4S��+6H2O���ʱ����Ϊ����������ȡʱ�Ľ�ȡ�ʣ�3MnO2 +2FeS2+12H+=3Mn2++2Fe3++4S��+6H2O��
(2)�������Ӿ��н�ǿ�Ļ�ԭ�ԣ����Ժ�����ط�Ӧ���ʼ����������ӣ��ɼӸ�����أ�����ɫ��ɫ�������������ӣ�Ҫ��ȥ�������ӣ����Լ�����������ȥ��Ҳ���Լ���������̽�������������Ϊ�����ӣ�������轫��Һ��������Ȼ���ڲ��Ͻ����¼�������pH4~5���ټ������һ��ʱ�䣬���������"��Ŀ���ƻ������������壬ʹ֮���������ڹ��˷��룬��˲������������Ϊ�����������ʱ����Ϊ��ȡ������Һ���Թ��У�������������Һ����ɫ��ȥ������H2O2��MnO2��Fe(OH)3��
(3)���������90~100���½��У���Ӧ�Ļ�ѧ����ʽΪ(NH4)2Sx+1=2NH3��+H2S��+xS�����ʱ����Ϊ��(NH4)2Sx+1=2NH3��+H2S��+xS����
(4) S2-��NH4+������ˮ�⣬NH4+ˮ������ԣ�S2-ˮ��ʼ��ԣ���Һ�������ȡ�����������백ˮ���볣������Դ�С���ʱ����Ϊ���Ƚ�SH-��NH4+ˮ�ⳣ������Դ�С��
(5)��֪��ʵ�������£����������Һ����������Һ��ϲ����������̣���Ӧ�����ӷ���ʽΪ
2![]() ��3Mn2+��2H2O��5MnO2����4H+��
��3Mn2+��2H2O��5MnO2����4H+��
n(KMnO4)=c mol/L��v��10-3L=cV��10-3mol����Ʒ��Mn2+����������Ϊ(1.5cv��10-3mol��55g/mol)/ag��100��=![]() ��100�����ʱ����Ϊ��
��100�����ʱ����Ϊ��![]() ��100����
��100����

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�