��Ŀ����
����Ŀ�����������Ǵ�����Ҫ��Ⱦ��ɲ���ǿ�����������ѳ����ȷֽ�ȷ��������������
����֪:
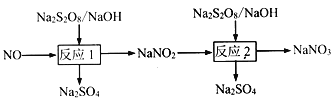
(1)д����Ӧ1�����ӷ���ʽ___________��
(2)�ڷ�Ӧ2��,NO2���ij�ʼŨ��Ϊ0.1mol��L��1,��ӦΪNO2��+S2O82��+2OH-![]() NO3��+2SO42��+H2O����ͬ�¶��£��ﵽƽ��ʱNO2�����ѳ������������(Na2S2O8)��ʼŨ���Ĺ�ϵ����ͼ��ʾ��
NO3��+2SO42��+H2O����ͬ�¶��£��ﵽƽ��ʱNO2�����ѳ������������(Na2S2O8)��ʼŨ���Ĺ�ϵ����ͼ��ʾ��

�ٱȽ�a��b��ķ�Ӧ����:va��_______vb��(���>����<����=��)
�������¶ȵ����ߣ��÷�Ӧ�Ļ�ѧƽ�ⳣ��K______(����������䡱��С��)��
����֪90��ʱ��Kw=3.6��10��13����b���Ӧ��pHΪ12,����¶���K=_____(����һλС��)��
(3)��ҵ��������ƺ�����Ļ��Һ�Ʊ���������(Na2S2O8),�����ĵ缫��ӦʽΪ_______��
��N2O�ڽ�۱��淢���ȷֽ⣺2N2O(g)=2N2(g)+O2(g) ��H��
�ش���������:
(4)��֪:2NH3(g)+3N2O(g)=4N2(g)+3H2O(1) ��H1
4NH3(g)+3O2(g)=2N2(g)+6H2O(1) ��H2
��H=_____��(����H1����H2�Ĵ���ʽ)
(5)ij�¶���,���c(N2O)��ʱ��t�仯��ϵ��ͼ��ʾ��
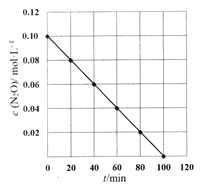
��֪˲ʱ��Ӧ����v��c(N2O)�Ĺ�ϵΪv=kcn(N2O)(k�Ƿ�Ӧ���ʳ���),��k=________,n=_____.
���𰸡� 2NO+S2O82��+4OH��![]() 2NO2��+2SO42��+2H2O < ���� 20.5L��mol��1 2SO42�D�D2e��=S2O82�� 2/3��H1-1/3��H2 0.0010mol��L-1��min-1 0
2NO2��+2SO42��+2H2O < ���� 20.5L��mol��1 2SO42�D�D2e��=S2O82�� 2/3��H1-1/3��H2 0.0010mol��L-1��min-1 0
����������1���������ӷ�Ӧ����ʽ����д����Ӧ1��ͨ��NO��Na2S2O8��NaOH��������Na2SO4��NaNO2������NO��Na2S2O8��NaOH��NaNO2��Na2SO4��NO��N�Ļ��ϼ��ɣ�2�ۡ���3�ۣ����ϼ�����1�ۣ�Na2S2O8�к��й��������ӣ����ϼ۽���2�ۣ����ݻ��ϼ����������õ����ӷ�Ӧ����ʽΪ2NO��S2O82����4OH��=2NO2����2SO42����2H2O����2������Ӱ�컯ѧ��Ӧ���ʵ����ء���ѧƽ�ⳣ���ļ��㣬��b��ij�ʼŨ�ȴ���a�㣬Ũ��Խ��Ӧ����Խ�죬��va��<vb�����ڸ���ͼ����ͬ��ʼŨ���£��¶�Խ�ߣ�NO2����ȥ����Խ�ߣ��������¶ȣ�ƽ��������Ӧ������У�����Ӧ�����ȷ�Ӧ����ѧƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬�������¶ȣ���ѧƽ�ⳣ��������pH=12����ʱc(OH��)=3.6��10��1mol��L��1��
NO2��+S2O82��+2OH-![]() NO3��+2SO42��+H2O��
NO3��+2SO42��+H2O��
��ʼ��0.1 0.2
�仯��0.09 0.09 0.09 0.18
ƽ�⣺0.01 0.11 3.6��10��1 0.09 0.18 ���ݻ�ѧƽ�ⳣ���Ķ��壬K= 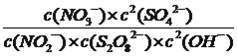 ��������ֵ���ó�K=20.5����3������缫��Ӧʽ����д�����ݵ缫��ԭ����������ʧȥ���ӣ����ϼ����ߣ����������ӦʽΪ2SO42����2e��=S2O82������4�������Ȼ�ѧ��Ӧ����ʽ�ļ��㣬 ��2NH3(g)+3N2O(g)=4N2(g)+3H2O(1)����4NH3(g)+3O2(g)=2N2(g)+6H2O(1)������Ŀ�귴Ӧ����ʽ��(2���٣���)/3�ó�����H=(2��H1����H2)/3����5�����黯ѧ��Ӧ���ʵļ��㣬����ͼ�������ͬʱ��Σ���ѧ��Ӧ������ȣ�ȡʱ��ֱ�Ϊ20��40������k��0.08n=k��0.06n�����n=0��0��20minʱ��N2O��ʾ�Ļ�ѧ��Ӧ������(0.10��0.08)/20mol/(L��min)=0.0010 mol/(L��min)������������ʽ���ó�k=0.0010 mol/(L��min)��
��������ֵ���ó�K=20.5����3������缫��Ӧʽ����д�����ݵ缫��ԭ����������ʧȥ���ӣ����ϼ����ߣ����������ӦʽΪ2SO42����2e��=S2O82������4�������Ȼ�ѧ��Ӧ����ʽ�ļ��㣬 ��2NH3(g)+3N2O(g)=4N2(g)+3H2O(1)����4NH3(g)+3O2(g)=2N2(g)+6H2O(1)������Ŀ�귴Ӧ����ʽ��(2���٣���)/3�ó�����H=(2��H1����H2)/3����5�����黯ѧ��Ӧ���ʵļ��㣬����ͼ�������ͬʱ��Σ���ѧ��Ӧ������ȣ�ȡʱ��ֱ�Ϊ20��40������k��0.08n=k��0.06n�����n=0��0��20minʱ��N2O��ʾ�Ļ�ѧ��Ӧ������(0.10��0.08)/20mol/(L��min)=0.0010 mol/(L��min)������������ʽ���ó�k=0.0010 mol/(L��min)��