��Ŀ����
19�� ��һ��2L���ܱ������У�������Ӧ2SO3��g��?2SO2��g��+O2��g������H��0������SO3�ı仯��ͼ��ʾ��
��һ��2L���ܱ������У�������Ӧ2SO3��g��?2SO2��g��+O2��g������H��0������SO3�ı仯��ͼ��ʾ����1��д���÷�Ӧ��ƽ�ⳣ������ʽ$\frac{{c}^{2}��S{O}_{2}����c��{O}_{2}��}{{c}^{2}��S{O}_{3}��}$��
��2����O2��ʾ0��8min�ڸ÷�Ӧ��ƽ������v=0.0125 mol/��L•min����
��3�������¶ȣ�Kֵ�����������������ƽ����Է�����������С�����������С�����䡱��
��4����8min��ѹ������Ϊ1L����SO3�ı仯����Ϊc
A��a B��b C��c D��d
��5��������¶��µ�ƽ�ⳣ��K=0.4 mol/L��
���� ��1����ѧƽ�ⳣ����ָ��һ���¶��£����淴Ӧ����ƽ��ʱ���������Ũ��ϵ������֮���뷴Ӧ���Ũ��ϵ������֮���ıȣ����塢��Һ�岻��Ҫ�ڻ�ѧƽ�ⳣ����д����
��2������v=$\frac{��c}{��t}$����v��SO3��������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��O2����
��3������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���ƽ�ⳣ����������������ʵ���������������������䣬���M=$\frac{m}{n}$�ж�ƽ����Է��������仯��
��4����8min��ѹ������Ϊ1L��˲��SO3�����ʵ������䣬ѹǿ����ƽ�������ƶ���SO3�����ʵ�������ƽ��״̬��ƽ��ʱС��0.6mol��
��5��8min����ƽ�⣬ƽ��ʱ��������Ϊ0.2mol������ƽ��ʱ��������ʵ���Ũ�ȣ�����ƽ�ⳣ������ʽ���㣮
��� �⣺��1����Ӧ2SO3��g��?2SO2��g��+O2��g���Ļ�ѧƽ�ⳣ������ʽK=$\frac{{c}^{2}��S{O}_{2}����c��{O}_{2}��}{{c}^{2}��S{O}_{3}��}$��
�ʴ�Ϊ��$\frac{{c}^{2}��S{O}_{2}����c��{O}_{2}��}{{c}^{2}��S{O}_{3}��}$��
��2��0��8min��������������Ϊ0.6mol-0.2mol=0.4mol����v��SO3��=$\frac{\frac{0.4mol}{2L}}{8min}$=0.025 mol/��L•min��������֮�ȵ��ڻ�ѧ������֮�ȣ�v��O2��=$\frac{1}{2}$v��SO3��=0.0125 mol/��L•min����
�ʴ�Ϊ��0.0125 mol/��L•min����
��3������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���ƽ�ⳣ����������������ʵ���������������������䣬��M=$\frac{m}{n}$��֪ƽ����Է���������С��
�ʴ�Ϊ������С��
��4����8min��ѹ������Ϊ1L��˲��SO3�����ʵ������䣬ѹǿ����ƽ�������ƶ���SO3�����ʵ�������ƽ��״̬��ƽ��ʱС��0.6mol��ͼ��������c���ϣ�
�ʴ�Ϊ��c��
��5��8min����ƽ�⣬ƽ��ʱ��������Ϊ0.2mol����
2SO3��g��?2SO2��g��+O2��g��
��ʼŨ�ȣ�mol/L����0.3 0 0
�仯Ũ�ȣ�mol/L����0.2 0.2 0.1
ƽ��Ũ�ȣ�mol/L����0.1 0.2 0.1
ƽ�ⳣ������ʽK=$\frac{{c}^{2}��S{O}_{2}����c��{O}_{2}��}{{c}^{2}��S{O}_{3}��}$=$\frac{0��{2}^{2}��0.1}{0��{1}^{2}}$ mol/L=0.4mol/L��
�ʴ�Ϊ��0.4 mol/L��
���� ���⿼�黯ѧƽ���йؼ��㼰Ӱ�����ء�ƽ�ⳣ������Ӧ���ʵȣ��ѶȲ���ע��ƽ�ⳣ������λ�뻯ѧ�������йأ�

| A�� | $\frac{��}{400}$ | B�� | $\frac{20}{��}$ | C�� | 2.5 �� | D�� | 1.25 �� |
| A�� | Ӧ�û��β������������������Һ | |
| B�� | ʵ����Ӧʹ����ͬŨ�Ⱥ����������������Һ��������Һ | |
| C�� | ʵ����������С��ͬ���ձ���Ҫ������ֽ����Ŀ���Ǽ���ʵ���������������ʧ | |
| D�� | ����ͬ�����Ũ�������������Һ����ʵ�飬����к��ȵ���ֵ������ֵƫ�� |
| A�� | 100mL 2 mol/L��������п��Ӧʱ�������������Ȼ�����Һ���������������ʲ��� | |
| B�� | ����Ƭ��ϡ���ᷴӦ��ȡ����ʱ��������Ƭ��Ũ������Լӿ�������������� | |
| C�� | ��������Ĵ�������һ�����ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�������Ӧ���ʼ��� | |
| D�� | ����β���е�CO��NO���Ի�����Ӧ����N2��CO2�������������Ӱ��CO��ת���� |
��KOH ��CuSO4��Al ��HCl �����ǣ�
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ۢ� | D�� | �٢ڢ� |
| A�� | NaOH��Һ�����ᷴӦ | B�� | KOH��Һ��ϡ���ᷴӦ | ||
| C�� | Ba��OH��2��Һ��ϡ���ᷴӦ | D�� | ����ʯ��ˮ��ϡ���ᷴӦ |
| A�� | ���� | B�� | HCl | C�� | NaOH��Һ | D�� | CuƬ |
| A�� | ��ƽ�й�����NaCl��Һ��Fe��OH��3����ʱ��������������ͬ | |
| B�� | һ������������ʱ�������пɷ��������ЧӦ | |
| C�� | ����������������ɢϵ�ı��������Ƿ�ɢ�ʵ���ֱ����1��100nm֮�� | |
| D�� | Al��OH��3�����ܹ�ʹˮ�������Ĺ�������������ﵽ��ˮĿ�� |
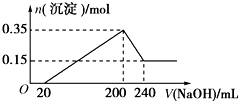 ��һ��������þ�������Ͷ��200mL�����У�����ȫ���ܽ����������Һ�м���5mol/L��NaOH��Һ�����ɳ��������ʵ���n�����NaOH��Һ�����V�ı仯��ͼ��ʾ��
��һ��������þ�������Ͷ��200mL�����У�����ȫ���ܽ����������Һ�м���5mol/L��NaOH��Һ�����ɳ��������ʵ���n�����NaOH��Һ�����V�ı仯��ͼ��ʾ��