��Ŀ����
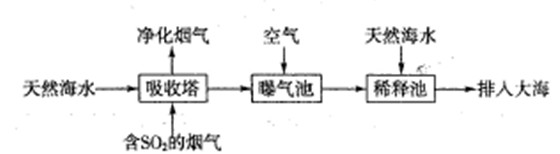
�������������Ű�ҹ��ֵ���������SO2����ɿ�������Ⱦ����Ҫԭ�������Ƽ�ѭ�����ɳ�ȥSO2��
��1���Ƽ�ѭ�����У�����ҺΪNa2SO3��Һ���÷�Ӧ�����ӷ���ʽ��________��
��2����֪H2SO3�ĵ��볣��ΪK1��1.54��10��2��K2��1.024��10��7��H2CO3�ĵ��볣��ΪK1��4.30��10��7��K2��5.60��10��11�������������Դ����������______������ţ���
A.CO32�� HSO3�� B. HCO3�� HSO3�� C. SO32�� HCO3�� D. H2SO3 HCO3��
��3������Һ����SO2�Ĺ����У�pH��n(SO32��):n(HSO3��)�仯��ϵ���±���
| n(SO32��):n(HSO3��) | 91:9 | 1:1 | 1:91 |
| pH | 8.2 | 7.2 | 6.2 |
�ٸ����ϱ��ж�NaHSO3��Һ��_______�ԣ��Խ���ԭ��__________________________��
����NaHSO3��Һ������Ũ�ȹ�ϵ����ȷ����_______������ţ���
A��c(Na+)��2c(SO32-)+c(HSO3-)
B��c(Na+)��c(HSO3-)��c(H+)��c(SO32-)��c(OH-)
C��c(H2SO3)+ c(H+)��c(SO32-)+c(OH-)
D��c(Na+)+ c(H+)��2c(SO32-)+ c(HSO3-)+ c(OH-)
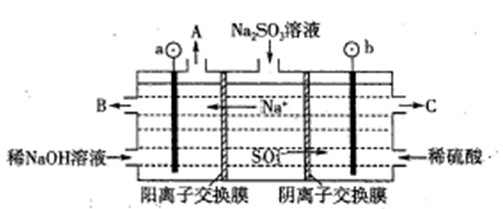
��4��������Һ��pH����ԼΪ6ʱ�����������۴�����ֱ���õ�pH��8������Һ��ѭ�����ã����ʾ��ͼ���£�
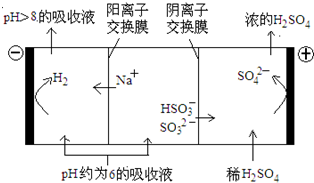
��д�������Ϸ����ĵ缫��Ӧʽ____________��
�ڵ��缫����1mol����ת��ʱ�������������Ϊ__________��
��1��SO32����SO2��H2O��2HSO3����1�֣�
��2��BC��2�֣�ȫ�Ե�2�֣��д����÷֣�ѡ��ȫ��1�֣�
��3�����ᣨ1�֣�����������������Һ��HSO3�����ڵ���ƽ�⣺HSO3�� SO32����H����ˮ��ƽ��HSO3����H2O
SO32����H����ˮ��ƽ��HSO3����H2O H2SO3��OH����HSO3���ĵ���̶�ǿ��ˮ��̶ȣ�����Һ�����ԣ�2�֣���A��1�֣�
H2SO3��OH����HSO3���ĵ���̶�ǿ��ˮ��̶ȣ�����Һ�����ԣ�2�֣���A��1�֣�
��4����HSO3����H2O��2e����SO42����3H����2�֣���1g��1�֣�
���������������1���������Ƕ�Ԫ���ᣬ������������SO2ת��Ϊ��ʽ�Σ������Ƽ�ѭ�����У�����ҺΪNa2SO3��Һʱ��Ӧ�����ӷ���ʽΪSO32����SO2��H2O��2HSO3����
��2������ĵ��볣��Խ������Խǿ�������H2SO3�ĵ��볣��ΪK1��1.54��10��2��K2��1.024��10��7��H2CO3�ĵ��볣��ΪK1��4.30��10��7��K2��5.60��10��11��֪������ǿ��˳����H2SO3��H2CO3��HSO3����HCO3�������ݽ�ǿ�����Ʊ����������֪��CO32����HSO3����Ӧ����HCO3����SO32�������ܴ������棻H2SO3��HCO3����Ӧ����CO2��HSO3�������ܴ������档��HCO3����HSO3����SO32����HCO3�������Դ������棬��ѡBC��
��3��������SO32��ֻ��ˮ�⣬��Һ�Լ��ԣ����Ը���n(SO32��):n(HSO3��)��1:91ʱ��Һ�����Կ�֪��NaHSO3��Һ�����ԡ�����������������������Һ��HSO3�����ڵ���ƽ�⣺HSO3�� SO32����H����ˮ��ƽ��HSO3����H2O
SO32����H����ˮ��ƽ��HSO3����H2O H2SO3��OH����HSO3���ĵ���̶�ǿ��ˮ��̶ȣ�����Һ�����ԡ�
H2SO3��OH����HSO3���ĵ���̶�ǿ��ˮ��̶ȣ�����Һ�����ԡ�
�ڸ��������غ��֪��c(Na+)��c(SO32-)+c(HSO3-)+ c(H2SO3)��A����ȷ��B������HSO3���ĵ���̶�ǿ��ˮ��̶ȣ��Ҿ��Ǻ����ģ�������Һ��c(Na+)��c(HSO3-)��c(H+)��c(SO32-)��c(OH-)��B��ȷ��C�����ݵ���غ��֪c(Na+)+ c(H+)��2c(SO32-)+ c(HSO3-)+ c(OH-)�������غ��֪c(Na+)��c(SO32-)+c(HSO3-)+ c(H2SO3)����Һ��c(H2SO3)+ c(H+)��c(SO32-)+c(OH-)��C��ȷ��D�����ϵ���غ�c(Na+)+ c(H+)��2c(SO32-)+ c(HSO3-)+ c(OH-)��D��ȷ����ѡA��
��4���ٵ������������Դ������������ʧȥ���ӣ�����������Ӧ������װ�ÿ�֪�������������ɣ�������Һ��pH��6����Һ����Ҫ����HSO3-���ڣ�����������Ҫ��HSO3-�ŵ磬��������Ϸ����ĵ缫��ӦʽΪHSO3����H2O��2e����SO42����3H����
�ڵ����������õ����裬�����װ�ÿ��ж���������Һ�е������ӷŵ������������缫��ӦʽΪ2H����2e����H2�������缫����1mol����ת��ʱ�����õ�0.5mol�����������������������Ϊ0.5mol��2g/mol��1g��
���㣺�����������ε����ʡ�����ˮ�⡢������ʵĵ��롢���볣����Ӧ�á���Һ������Ũ�ȴ�С�Ƚ��Լ��绯ѧԭ����Ӧ�������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���֪25 ��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��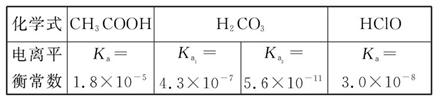
�ش��������⣺
(1)���ʵ���Ũ�Ⱦ�Ϊ0.1 mol��L-1��������Һ��
a.CH3COONa b.Na2CO3 c.NaClO d.NaHCO3
pH��С��������˳���� (�ñ����д)��
(2)�����£�0.1 mol/L��CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ����� ��
| A��c(H+) |
| B��c(H+)/c(CH3COOH) |
| C��c(H+)��c(OH-) |
| D��c(OH-)/c(H+) |
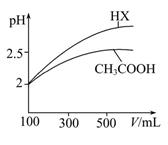
(3)�����Ϊ100 mL pH��2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����HX�ĵ���ƽ�ⳣ�� (����ڡ�����С�ڡ����ڡ�)CH3COOH�ĵ���ƽ�ⳣ���������� ��
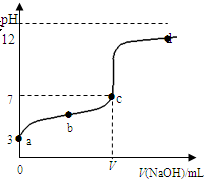
�ڳ�������20mL 0.1 mol��L��1ij�ᣨ��HAc��ʾ����Һ����μ���0.1 mol��L��1 NaOH��Һ����pH�����ⶨ��Һ��pH�������±�������pH��NaOH��Һ�����ϵ��������ͼ��ʾ�������¶ȱ仯��������ݱ������ݺ͵ζ����ش��������⣺
| V(NaOH)mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 | 40.00 |
| pH | 3.0 | 4.7 | 5.7 | 6.7 | 7.7 | 8.7 | 9.7 | 10.7 | 11.7 | 12.5 |
��2����V (NaOH)��20.00mLʱ����д����Һ����Ҫ���ڵ�����ƽ��״̬�ı�ʾʽ_______________��________________����Һ������Ũ���ɴ�С��˳����___________________��
��3��a��b��c��d�ĵ��Ӧ��Һ��ˮ�ĵ���̶��ɴ�С��˳����_________________��
��4�����¶��£��ζ�������c��ʱHAc�ĵ���ƽ�ⳣ��Ka=_______���ú�V�Ĵ�����ʽ��ʾ����
ijѧ����0.10 mol/L��NaOH��Һ�ζ�ijŨ�ȵ����ᡣ��¼�������£�
| ʵ����� | ����Һ�����mL�� | ������NaOH��Һ�������mL�� | |
| �ζ�ǰ | �ζ��� | ||
| 1 | 20.00 | 0.50 | 20.54 |
| 2 | 20.00 | 6.00 | 26.00 |
| 3 | 20.00 | 1.40 | 21.36 |
��1���ζ�ʱѡ�÷�̪��Һ��ָʾ��������жϵζ��ﵽ�յ� ��
��2����������ʵ���Ũ��Ϊ_____________
��3����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ���Բⶨ�����Ӱ���� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����ͬ����
��4��ijͬѧ����֪ȷŨ�ȵĸ��������Һ�ζ���Һ��Fe2+��Ũ�ȣ����������ҺӦʢ���� �У���ס����ҡ������÷�Ӧ�����ӷ���ʽΪ�� ��

��ҵ���õ�ⱥ��NaCl��Һ�ķ�������ȡNaOH��Cl2��H2����������Ϊԭ������һϵ�л�����Ʒ����Ϊ�ȼҵ��
��1����������Ĥ��������ʳ��ˮʱ��Cl2����NaOH��ֽӴ������²������NaClO��H2����Ĥ��������ʳ��ˮ��Ӧ�����ӷ���ʽΪ ��
��2���ȼҵ���ܸߣ�һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խڣ��磩��30�����ϡ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ����
�پ����Ƶı���NaCl��ҺӦ��ͼ�е��ص� ����д���� ���ҡ�����ע�롣
��ͼ��X��____ __���ѧʽ�����������ҵĵ缫��ӦʽΪ�� _ ��ͼʾ������������Һ��������a����b���Ĺ�ϵ�� ������ĸ����
A. a%=b% B. a%��b% C. a%��b%
�ۼ��е����ӽ���ĤΪ ��������ӽ���Ĥ���������ӽ���Ĥ������
��3���ȼҵ�IJ���NaOH�벻ͬ���ʷ�Ӧ�������ɲ�ͬ���Ρ���֪�����£�Ũ�Ⱦ�Ϊ0.1 mol/L��4��������ҺpH���±���
| ���� | Na2CO3 | NaHCO3 | NaClO | NaHSO3 |
| pH | 11.6 | 9.7 | 10.3 | 5.2 |
����˵���У�����ȷ���� ������ĸ��
a������ˮ�м���NaHCO3������������ˮ�д������Ũ��
b��������Һ�У�ˮ�ĵ���̶�������NaClO
c�������£���ͬ���ʵ���Ũ�ȵ�H2SO3��H2CO3��HClO��pH������H2SO3
d��NaHSO3��Һ������Ũ�ȴ�С˳��Ϊc��Na+��> c��H+��>c��HSO3-�� >c��SO32-��>c��OH-��
ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2����Pb(OH)����Pb(OH)2��Pb(OH)3-��Pb(OH)42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ�� 
(1)Pb(NO3)2��Һ�У� ________2(�>����������<��)��������Һ�е����Ȼ����Һ��
________2(�>����������<��)��������Һ�е����Ȼ����Һ�� ���ӣ����ܵ�ԭ����________________________________��
���ӣ����ܵ�ԭ����________________________________��
(2)��Pb(NO3)2��Һ�е���ϡNaOH��Һ��pH��8ʱ��Һ�д��ڵ�������(Na������)��__________��pH��9ʱ��Ҫ��Ӧ�����ӷ���ʽΪ_______________________��
(3)ij�������Ʊ���һ��������Ǧ��������Чȥ��ˮ�еĺ���Ǧ��ʵ�������±���
| ���� | Pb2�� | Ca2�� | Fe3�� | Mn2�� | Cl�� |
| ����ǰŨ��/(mg��L��1) | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ��/(mg��L��1) | 0.004 | 22.6 | 0.040 | 0.053 | 49.8 |
(4)�������Ǧ��(��EH��ʾ)��Ǧ��Ҫ�����ķ�Ӧ������Ϊ��2EH(s)��Pb2��
 E2Pb(s)��2H������Ǧ�������pH��ΧΪ( )
E2Pb(s)��2H������Ǧ�������pH��ΧΪ( )A��4��5 B��6��7 C��9��10 D��11��12
 Fe3+(aq)+3OH-(aq) �� ��H=" a" kJ?mol-1
Fe3+(aq)+3OH-(aq) �� ��H=" a" kJ?mol-1