��Ŀ����
5��д�����ӷ���ʽ����1��H2Sͨ���KMnO4��Һ��Ӧ��2MnO4-+5H2S+6H+=2Mn2++5S��+8H2O��
��2��Fe2��SO4��3��Һ��ͨ��SO2���壺SO2+2Fe3++2H2O=2Fe2++SO42-+4H+��
��3����FeBr2��Һ��ͨ������ʵ�����������2Cl2+2Br-+2Fe2+=4Cl-+Br2+2Fe3+��
��4�������ʯ��ˮ�м������NaHCO3 ����ҺCa2++2OH-+2HCO3-=CaCO3��+CO32-+2H2O��
��5����AlCl3 ��Һ�м�������NaOH����Һ��Al3++40H-�TAlO2-+2H2O��
���� ��1��H2Sͨ���KMnO4��Һ��Ӧ����������ԭ��Ӧ���������������Ӻ�ˮ��
��2�������������ܹ�������������������������ӣ�
��3�����������ӻ�ԭ��ǿ�������ӣ��������������������ӣ������������ӣ�
��4��̼�����ƹ�������Ӧ����̼��Ƴ�����̼���ƺ�ˮ��
��5����Ӧ����ƫ�����ƺ�ˮ��
��� �⣺��1��H2Sͨ���KMnO4��Һ��Ӧ�����ӷ���ʽ��2MnO4-+5H2S+6H+=2Mn2++5S��+8H2O��
�ʴ�Ϊ��2MnO4-+5H2S+6H+=2Mn2++5S��+8H2O��
��2��Fe2��SO4��3��Һ��ͨ��SO2���壬���ӷ���ʽ��SO2+2Fe3++2H2O=2Fe2++SO42-+4H+��
�ʴ�Ϊ��SO2+2Fe3++2H2O=2Fe2++SO42-+4H+��
��3����FeBr2��Һ��ͨ������ʵ��������������ӷ���ʽ��2Cl2+2Br-+2Fe2+=4Cl-+Br2+2Fe3+��
�ʴ�Ϊ��2Cl2+2Br-+2Fe2+=4Cl-+Br2+2Fe3+��
��4�������ʯ��ˮ�м������NaHCO3 ����Һ�����ӷ���ʽ��Ca2++2OH-+2HCO3-=CaCO3��+CO32-+2H2O��
�ʴ�Ϊ��Ca2++2OH-+2HCO3-=CaCO3��+CO32-+2H2O��
��5����AlCl3 ��Һ�м�������NaOH����Һ�����ӷ���ʽ��Al3++40H-�TAlO2-+2H2O��
�ʴ�Ϊ��Al3++40H-�TAlO2-+2H2O��
���� ���⿼�����ӷ�Ӧ����ʽ��д�����շ����ķ�Ӧ�����ӷ�Ӧ����д����Ϊ���Ĺؼ�������������ԭ��Ӧ�������йص����ӷ�Ӧ�Ŀ��飬��Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 115��Ԫ��˥���113��Ԫ���ǻ�ѧ�仯 | |
| B�� | 115��Ԫ��X�Ľ������ԭ������Ϊ288 | |
| C�� | 113��Ԫ�ص�ԭ���������3������ | |
| D�� | ��115��Ԫ����NԪ��ͬ���壬�����Ƿǽ���Ԫ�� |
| A�� | y=1•x% | B�� | y=��1-1•x%�� | C�� | y=��1-1•x%����0.5 | D�� | y=��1-1•x%����0.5 |
| A�� | Na2O��Na2O2�������Ӻ������ӵĸ����ȷֱ�Ϊ2��1��1��1 | |
| B�� | Na2CO3��Һ��NaHCO3��Һ���ܸ�CaCl2��Һ��Ӧ�õ���ɫ���� | |
| C�� | Na2CO3��Һ����Ca��OH��2��Ӧ������ɫ��������NaHCO3��Һ���� | |
| D�� | Na2O2��������������Na2O���� |
| A�� | CH2�TCH2+3O2$��_{��}^{����}$2CO2+2H2O | |
| B�� | CH2�TCH2+H2$\stackrel{����}{��}$CH3-CH3 | |
| C�� | CH2�TCH2+Br2��CH2Br-CH2Br | |
| D�� | CH2�TCH2+H2O$��_{���ȼ�ѹ}^{����}$CH3CH2OH |
| A�� | �����¶� | B�� | ʹ�ô��� | C�� | �ı���ϵѹǿ | D�� | �ı�����Ũ�� |
| A�� | ${\;}_{b}^{a}{X}^{n-}$���е�������Ϊb+a | B�� | ${\;}_{b}^{a}{X}^{n-}$���еĵ�����Ϊa-n | ||
| C�� | Xԭ�ӵ�������Ϊa+b+n | D�� | 1��Xԭ�ӵ�����ԼΪ$\frac{a}{6.02��1{0}^{23}}$g |
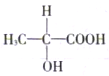 �����и������ᣬ������ӵĽṹ��ʽ��ͼ��ʾ��ش�
�����и������ᣬ������ӵĽṹ��ʽ��ͼ��ʾ��ش�