��Ŀ����
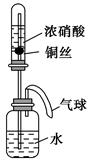
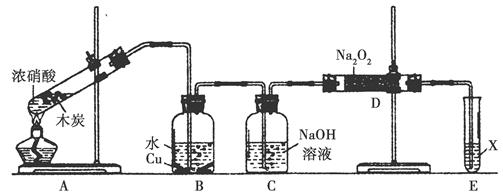
ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
��̼���� ��̼������ ��̼����� ���Ȼ�� ����ʯ�� ����������

����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ƣ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1����A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ�� ����������ţ�
��2��Bװ�õ�����Ϊ
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ ��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е� �������и�����ţ�
��4��ͼE�г���ͨ������������Ϊ ��
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸�������� ��
��̼���� ��̼������ ��̼����� ���Ȼ�� ����ʯ�� ����������

����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ƣ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1����A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ�� ����������ţ�
��2��Bװ�õ�����Ϊ
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ ��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е� �������и�����ţ�
| A��Na2CO3 | B��AgNO3 | C��H2SO4 | D��FeSO4 |
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸�������� ��
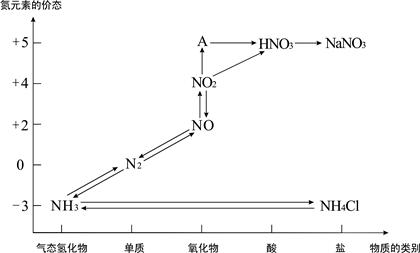
��1���ۣ�2�����ն�����̼��ˮ��������������
��3��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O B C
��4��ʹ�к��������ո���ȫ
��5��Dװ�õ�Һ�������벣����C�У�ʹ���������ѣ���Cװ����Dװ��֮������һ������װ�á�
��3��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O B C
��4��ʹ�к��������ո���ȫ
��5��Dװ�õ�Һ�������벣����C�У�ʹ���������ѣ���Cװ����Dװ��֮������һ������װ�á�
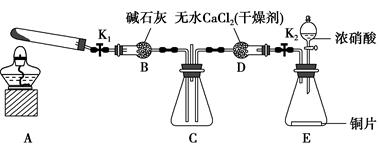
����������������װ��ͼ��C��D�з�Ӧ����֪����ʵ��Ϊ̽�����Ĵ�������������ͭ�ķ�Ӧ��װ��A������Ϊ���ɰ�����װ��B������Ϊ��Ӧ�ṩ������װ��E��FΪβ������װ�á���1��������������֪��A�����ɵ������к��а����������Ʒ�Ӧ�������������������̼��ˮ������A����ȡ����ʱֻ����һ��ҩƷ������ѡ���֪һ���Լ����ɰ����Ͷ�����̼���Լ�ѡ��̼����泥���Ϊ���ۣ���2��Bװ�������ù�����������̼����立ֽ����ɵĶ�����̼��ˮ����������������Ϊ�����ն�����̼��ˮ������������������3��D�з�ӦΪͭƬ����ϡ������������ͭ��һ��������ˮ�����ӷ���ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��ʹCuƬ�ܽ�����ʼӿ죬��������ԭ���ԭ������������Ũ�ȵķ�����A��Na2CO3 �������ᣬ����Ũ�ȼ�С����Ӧ���ʼ���������B��AgNO3 ��ͭ��Ӧ��������ͭ������ϡ������Һ������ԭ��ؼӿ췴Ӧ���ʣ���ȷ��C��H2SO4 ������������Ũ�ȼӿ�ͭ��ϡ����ķ�Ӧ���ʣ���ȷ��D��FeSO4����������ܼӿ�ͭ�ķ�Ӧ���ʣ�����ѡBC����4��ͼE�г���ͨ��������������ʹ���ɵ�һ����������ȫ��ת��Ϊ���������ȫ���գ���Ϊ��ʹ�к��������ո���ȫ����5��Dװ���е�Һ������������Cװ�ã���Ҫ��CD֮���һ����������װ�ã���Ϊ��Dװ�õ�Һ�������粣����C�У�ʹ���������ѣ���C��Dװ��֮������һ����ֹ������װ�á�

��ϰ��ϵ�д�
 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�
�����Ŀ
 CO2 (g)�� H2 (g) �� ��֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)������ ��
CO2 (g)�� H2 (g) �� ��֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)������ ��