��Ŀ����
����Ŀ����1��3.1 g Na2X����Na�� 0.1 mol����Na2X��Ħ������Ϊ________��X�����ԭ������Ϊ________��
��2��ͬ��ͬѹ�£�ͬ����ļ��������������֮��Ϊ_______��ԭ�Ӹ���֮��Ϊ_____������֮��Ϊ_________��������֮��Ϊ________��
��3���õ������0.1 mol��L��1��BaCl2��Һ����ʹ��ͬ�����Fe2(SO4)3��Na2SO4��Al2(SO4)3������Һ�е�SO42-��ǡ����ȫ�����������������ε����ʵ���Ũ��֮��Ϊ_____��
���𰸡�62g/mol 16 1��1 5��2 8��1 5��1 1��3��1
��������
��1������M=m/n���м��㣻
��2��ͬ��ͬѹ�£����֮�ȵ������ʵ���֮�ȣ��ٸ���ԭ�ӽṹ���м��㣻
��3������SO42+Ba2+=BaSO4�����м��㡣
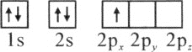
��1��һ�� Na2X���ж���Na�� ��0.1 mol Na��ʱ��Na2X�����ʵ���Ϊ0.05mol��������Ϊ3.1 g����M=m/n=3.1/0.05=62g/mol��X�����ԭ������=62-23![]() 2=16��
2=16��
��2��ͬ��ͬѹ�£����֮�ȵ������ʵ���֮�ȣ���ͬ����ļ��������������֮��Ϊ1��1��һ����������к���5��ԭ�ӣ�һ�����������к���2��ԭ�ӣ���ͬ����ļ��������ԭ�Ӹ���֮��Ϊ5��2�������Ħ������Ϊ16g/mol��������Ħ������Ϊ2g/mol�����ʵ������ʱ������֮��Ϊ16��2=8��1����������к��еĵ�����Ϊ10�������к��еĵ�����Ϊ2���������֮��Ϊ5��1��
��3������SO42+Ba2+=BaSO4�����������0.1 mol��L��1��BaCl2��Һ��n��Ba2+����ͬ�����ĵ�n��SO42����ͬ����n[Fe2(SO4)3]�� n��Na2SO4���� n[Al2(SO4)3]=![]() ��1��
��1��![]() =1��3��1��
=1��3��1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ͼ��ʾװ�ý�������ʵ�飺��������Һ�������У�Ԥ���������ʵ���������

ѡ�� | �������� | �������� | Ԥ�����е����� |
A�� | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
B�� | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
C�� | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
D�� | ������Һ | �������������Һ | ��Һ����ɫ |