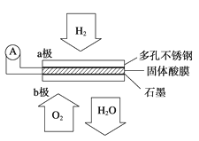
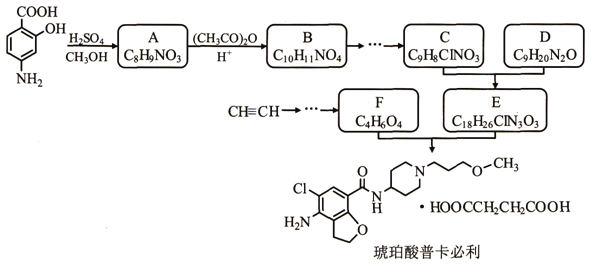
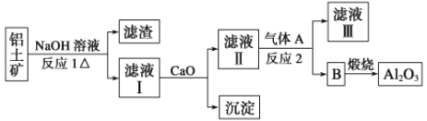
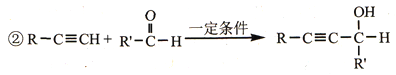��Ŀ����
����Ŀ����ú��ʯ���п�����������ԭ��A��B��A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��һ�ֱ�ˮ�����״Һ�壬B����̼������Ԫ����ɣ�̼Ԫ������Ԫ�ص�������Ϊ12��1��B����Է�������Ϊ78���ش��������⣺
![]() �ĵ���ʽ______��A�Ľṹ��ʽ______��
�ĵ���ʽ______��A�Ľṹ��ʽ______��
![]() ��A���ڵ�ͬϵ��Cʹ������Ȼ�̼��Һ��ɫ�Ļ�ѧ��Ӧ����ʽ_________����Ӧ����______��
��A���ڵ�ͬϵ��Cʹ������Ȼ�̼��Һ��ɫ�Ļ�ѧ��Ӧ����ʽ_________����Ӧ����______��
![]() �ڵ�ˮ�м���B���ú������______��
�ڵ�ˮ�м���B���ú������______��
![]() ��Ũ������Ũ������
��Ũ������Ũ������![]() ��Ӧ�Ļ�ѧ��Ӧ����ʽ_____________����Ӧ����______��
��Ӧ�Ļ�ѧ��Ӧ����ʽ_____________����Ӧ����______��
![]() ��������A��B��ȫȼ��ʱ����
��������A��B��ȫȼ��ʱ����![]() �����ʵ���______
�����ʵ���______![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
���𰸡�
![]()
![]() �ӳɷ�Ӧ ��Һ�ֲ㣬�²���ɫ���ϲ��Ϻ�ɫ
�ӳɷ�Ӧ ��Һ�ֲ㣬�²���ɫ���ϲ��Ϻ�ɫ ![]() +HNO
+HNO 3+H2O ȡ����Ӧ
3+H2O ȡ����Ӧ ![]()
��������
A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪ![]() ��B��һ�ֱ�ˮ�����״Һ�壬B����̼������Ԫ����ɣ�̼Ԫ������Ԫ�ص�������Ϊ12��1����ԭ�Ӹ���֮��Ϊ1��1������
��B��һ�ֱ�ˮ�����״Һ�壬B����̼������Ԫ����ɣ�̼Ԫ������Ԫ�ص�������Ϊ12��1����ԭ�Ӹ���֮��Ϊ1��1������![]() ��B����Է�������Ϊ78����
��B����Է�������Ϊ78����![]() ������ó�n=6������BΪ�����ݴ˽��
������ó�n=6������BΪ�����ݴ˽��
![]() ��������������A�ĵ���ʽΪ
��������������A�ĵ���ʽΪ ��A�Ľṹ��ʽΪ��
��A�Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ�� ��
��![]() ��
��
![]() ���ڵ�ͬϵ��CΪ
���ڵ�ͬϵ��CΪ![]() ��ʹ��ˮ��ɫ�����ӳɷ�Ӧ���÷�ӦΪ��
��ʹ��ˮ��ɫ�����ӳɷ�Ӧ���÷�ӦΪ��![]() ��
��
�ʴ�Ϊ��![]() ���ӳɷ�Ӧ��
���ӳɷ�Ӧ��
![]() ��ˮ�м��뱽��ȡ�������ܶȱ�ˮ��С�������ϲ㣬�۲쵽�²���ɫ���ϲ��Ϻ�ɫ��
��ˮ�м��뱽��ȡ�������ܶȱ�ˮ��С�������ϲ㣬�۲쵽�²���ɫ���ϲ��Ϻ�ɫ��
�ʴ�Ϊ����Һ�ֲ㣬�²���ɫ���ϲ��Ϻ�ɫ��
![]() ��Ũ
��Ũ![]() ��Ũ
��Ũ![]() ��
��![]() ��Ӧ�Ļ�ѧ��Ӧ����Ϊ��
��Ӧ�Ļ�ѧ��Ӧ����Ϊ��![]() ������ȡ����Ӧ��
������ȡ����Ӧ��
�ʴ�Ϊ��
![]() �������������ĵ���Ԫ����������Խ����ͬ����������������Խ�࣬��ϩ��HԪ�����������ȱ���HԪ��������������ͬ��������ϩ����ȼ�գ���ϩ���ĵ��������࣬����������A��B��ȫȼ��ʱ����
�������������ĵ���Ԫ����������Խ����ͬ����������������Խ�࣬��ϩ��HԪ�����������ȱ���HԪ��������������ͬ��������ϩ����ȼ�գ���ϩ���ĵ��������࣬����������A��B��ȫȼ��ʱ����![]() �����ʵ���
�����ʵ���![]() ��
��
�ʴ�Ϊ��![]() ��
��

����Ŀ��Ϊ̽��Fe(NO3)2���������ȷֽ����Ͳ�������ʣ�ij��ѧС�鿪չ����̽����
���������ϣ�2KNO3![]() 2KNO2+O2�� Fe(NO3)2
2KNO2+O2�� Fe(NO3)2![]() FexOy+NO2��+O2��
FexOy+NO2��+O2��
ʵ��һ��̽��Fe(NO3)2�ȷֽ�����������Ԫ�صļ�̬����С���ͬѧ���ֽ��Ĺ����������������ϡH2SO4�õ���Ӧ������Һ����������̽��ʵ�顣
��1�����ᴿ���룩
����һ����Ԫ��ֻ��+2�ۣ�
���������Ԫ��_____________��
����������Ԫ�ؼ���+2������+3�ۡ�
��ʵ�����������һ����Һ�е���KSCN��Һ������һ����Һ�е�������KMnO4ϡ��Һ��
��2����ʵ������ʵ����_____________________��ʵ����____________________��
��3����ʵ����ۣ��������������Fe(NO3)2�ֽ�Ļ�ѧ����ʽ��_________________��
ʵ�����
��4��̽��Fe(NO3)2�ȷֽ������������ʡ�С����ͬѧ����������ʵ�飬�����ʵ���ȱ�������ݡ���ѡ�Լ�����Ʒ��ŨH2SO4��Һ��4mol/LNaOH��Һ��0.1mol/LBaCl2��Һ�������ǵ�ľ����0.1mol/L����KMnO4��Һ������ˮ��
ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ����Fe(NO3)2�������Թ��У����ȷֽ⡣ | ____________________________________��˵���ֽ�����������к���NO2�� |
����2������������������ͨ��ʢ������_________________��Ũ�����ϴ��ƿ��______________________�����һ�����ڼ��顣 | _____________________________________��˵���ֽ�����������к�O2�� |
ʵ������KNO3�л���Fe(NO3)2��Ϊȷ��������Ԫ�صĺ�����С���ͬѧ��������ʵ�飺��ȡ�������Ʒ10g����ּ��ȷֽ⣻������������ܽ⡢���ˣ�ȡ��������ϴ�ӡ�����Ƶ�������Ϊ3.2g������������Ԫ�ص���������Ϊ__________________����������λ��Ч���֣����ԭ��������Fe��56 O��16��