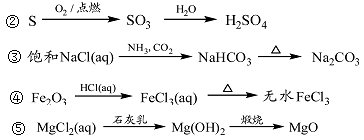
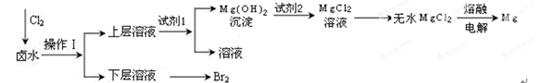
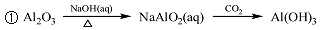��Ŀ����
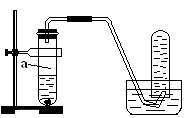
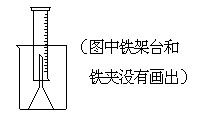
27. ��ͼ��ʾ����ѧ��ѧʵ���г�����װ�ã����ж�����;��
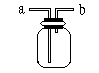
����ƿ��װ��X��Һ����CO��CO2�Ļ��������a�ܿ�ͨ�룬���Գ�ȥCO2����XΪ________��
��������ˮ���ռ�H2����H2����Ӧ��_______�����ţ���ͬ���ܿڵ��룻�����ſ������ռ�CO2����CO2����Ӧ��________ �ܿڵ��롣
��ҽԺ�����������ʱ��������������ƿ�벡�˺������֮�䰲װ��ˮ�ĸ�װ�ã��۲����ݲ�����������Ա���ڹ������ʣ���ʱ����Ӧ�� �ܿڵ��롣
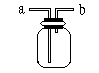
����ƿ��װ��X��Һ����CO��CO2�Ļ��������a�ܿ�ͨ�룬���Գ�ȥCO2����XΪ________��
| A��H2SO4 | B��NaOH | C��NaCl | D��HCl |
��ҽԺ�����������ʱ��������������ƿ�벡�˺������֮�䰲װ��ˮ�ĸ�װ�ã��۲����ݲ�����������Ա���ڹ������ʣ���ʱ����Ӧ�� �ܿڵ��롣
��1��B����2��b��a����3��a
��1��CO2������������ü�Һ���գ���ѡB��
��2���������ܶ�С�ڿ����ģ�����Ӧ����b��a����CO2���ܶȴ��ڿ�������Ӧ����a��b����
��3��Ҫ��۲쵽���ݣ��������Ӧ�ò��뵽��Һ�У���a����
��2���������ܶ�С�ڿ����ģ�����Ӧ����b��a����CO2���ܶȴ��ڿ�������Ӧ����a��b����
��3��Ҫ��۲쵽���ݣ��������Ӧ�ò��뵽��Һ�У���a����

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�����Ŀ