��Ŀ����
����Ŀ�������£���ijһԪ��![]() �ס��ҡ�������������ͬ��һԪ��
�ס��ҡ�������������ͬ��һԪ��![]() ��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH�����ʾ��
��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH�����ʾ��
ʵ�� ��� | HA�����ʵ� ��Ũ�� | NaOH�����ʵ� ��Ũ�� | ��Ϻ���Һ��pH |
�� |
|
| pH |
�� |
|
| pH |
�� |
|
|
|
�� |
|
| pH |
![]() �Ӽ��������������aֵ��С����ж�HA��ǿ�ỹ�����________________________________________________________________________��
�Ӽ��������������aֵ��С����ж�HA��ǿ�ỹ�����________________________________________________________________________��
![]() ��������Һ��
��������Һ��![]() ��
��![]() �Ĵ�С��ϵ��________
�Ĵ�С��ϵ��________![]() ����
����![]() ��
��
A.ǰ�ߴ� ![]() ���ߴ�
���ߴ�
C.������� ![]() ���ж�
���ж�
![]() �ӱ���ʵ�����������û����Һ��
�ӱ���ʵ�����������û����Һ��![]() __________
__________![]() ����
����![]() ����
����![]() ������
������![]() ��
��![]() ��
��
![]() ��������ʵ�����ݣ�д���û����Һ��������ʽ�ľ�ȷ���
��������ʵ�����ݣ�д���û����Һ��������ʽ�ľ�ȷ���![]() ��ʽ
��ʽ![]() ��
��![]() __________
__________![]() ��
��
���𰸡�![]() ʱ��HA��ǿ�
ʱ��HA��ǿ�![]() ʱ��HA������
ʱ��HA������ ![]()
![]()
![]()
��������
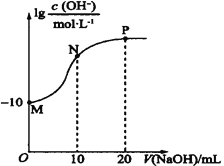
��1�������ʵ������ʱ������ǡ�÷�Ӧ�����Σ�������Һ��pH�ж�����ǿ����
��2���κ���Һ�ж����ڵ���غ㣬���ݵ���غ��жϣ�
��3�������Һ������Ϊ�����ʵ�����HA��NaA��pH>7˵��A-��ˮ�����HA�ĵ��룬��ϵ���غ��жϣ����������غ��2c(Na+)=c(A-)+c (HA)��
��4���ɵ���غ��ϵʽ���ε�c(Na+)-c(A-)=c(OH-)-c(H+)��
(1)��HA��ǿ�ᣬǡ����NaOH��Һ��Ӧ����ǿ��ǿ���Σ�pH=7����HA�����ᣬ���ɵ�NaAˮ���Լ��ԣ�pH>7��
�ʴ�Ϊ��a=7ʱ��HA��ǿ�a>7ʱ��HA�����
(2)�����Һ�д��ڵ���غ�c(Na+)+c(H+)=c(A)+c(OH)������pH=7����c(Na+)=c(A)��
�ʴ�Ϊ��C��
(3)�����Һ������Ϊ�����ʵ�����HA��NaA��pH>7˵��A��ˮ�����HA�ĵ��룬��������Ũ���ɴ�С��˳��Ϊc(Na+)>c(A)>c(OH)>c(H+)�����������غ��2c(Na+)=c(A)+c (HA)=0.2molL1��
�ʴ�Ϊ��c(Na+)>c(A)>c(OH)>c(H+)��0.2��
(4)�ɵ���غ��ϵʽ���ε�c(Na+)c(A)=c(OH)c(H+)=(1041010)molL1��
�ʴ�Ϊ��1041010��

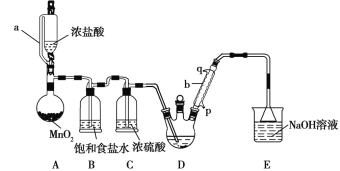
����Ŀ�������� ![]() ��һ����Ҫ�Ļ���ԭ�ϣ�һ���Ժ�������
��һ����Ҫ�Ļ���ԭ�ϣ�һ���Ժ�������![]() ��NiS��
��NiS��![]() ��FeO��CaO��
��FeO��CaO��![]() Ϊԭ����ȡ�������Ĺ�������ͼ����ͼ��ʾ��
Ϊԭ����ȡ�������Ĺ�������ͼ����ͼ��ʾ��
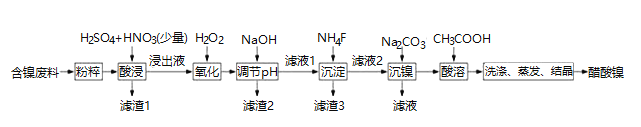
��������������������pH��������ʵ��ܽ��������
�������� | ��ʼ����ʱ��pH | ������ȫʱ��pH | ���� |
|
|
|
|
| �� |
|
|
|
| ���� |
|
|
|
| ���� |
|
|
|
|
|
![]() �ĵ���ʽΪ_________________��
�ĵ���ʽΪ_________________��
![]() ����pH�����У���ҺpH�ĵ��ڷ�Χ��_________________��
����pH�����У���ҺpH�ĵ��ڷ�Χ��_________________��
![]() ����1������3��Ҫ�ɷֵĻ�ѧʽ�ֱ���_________________��_________________��
����1������3��Ҫ�ɷֵĻ�ѧʽ�ֱ���_________________��_________________��
![]() ��������У�lmolNiSʧȥ
��������У�lmolNiSʧȥ![]() �����ӣ�ͬʱ����������ɫ�ж����壬д���÷�Ӧ�Ļ�ѧ����ʽ��________��
�����ӣ�ͬʱ����������ɫ�ж����壬д���÷�Ӧ�Ļ�ѧ����ʽ��________��
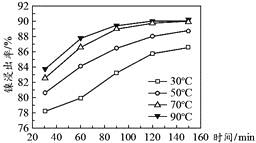
![]() ���������������䣬�ڲ�ͬ�¶��¶Ժ������Ͻ������������������ʱ��仯����ͼ��ʾ�����������¶���ʱ��ֱ�Ϊ_________________��
���������������䣬�ڲ�ͬ�¶��¶Ժ������Ͻ������������������ʱ��仯����ͼ��ʾ�����������¶���ʱ��ֱ�Ϊ_________________��
![]() ������������
���������У���![]() ����ʹ100mL����Һ�е�
����ʹ100mL����Һ�е�![]() ������ȫ
������ȫ![]() ������Ҫ����
������Ҫ����![]() �������������Ϊ___________
�������������Ϊ___________![]() ����С�����2λ��Ч����
����С�����2λ��Ч����![]() ��
��
![]() �����������ط�չ�ܿ죬������
�����������ط�չ�ܿ죬������![]() ��
��![]() �и�Ԫ�ػ��ϼ۾��ɿ�������
�и�Ԫ�ػ��ϼ۾��ɿ�������![]() ��ɣ�
��ɣ�![]()
![]()
![]() ���õ�طŵ�ʱ��������Ӧ��________��
���õ�طŵ�ʱ��������Ӧ��________��