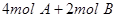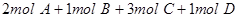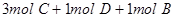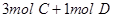��Ŀ����
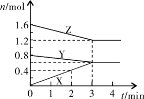
(13��)��100��ʱ����0.100 mol ������������������� 1 L ������յ��ܱ������У���һ��ʱ��Ը������ڵ�����Ũ�Ƚ��з����õ��±����ݣ�
(1)�÷�Ӧ��ƽ�ⳣ������ʽΪ______________________��
�ӱ��з�����c1________c2��c3________c4(�>������<������)��
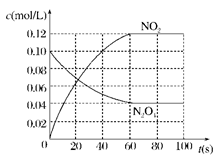
(2)����ͼ�л����������˷�Ӧ��c(N2O4)��c(NO2)��Ũ����ʱ��仯�����ߡ�
(3)�����������£��ӷ�Ӧ��ʼֱ���ﵽ��ѧƽ��ʱ��������������ƽ����Ӧ����Ϊ__ mol/(L��s)��
(4)��Ӧ�ﵽƽ���,���������ĸı��ʹNO2�����Ũ���������
A�������������ݻ������� B���ٳ���һ������N2O4
C�������һ������NO2 D���ٳ���һ������He
| ʱ��(s) | 0 | 20 | 40 | 60 | 80 |
| c(N2O4)(mol/L) | 0.100 | c1 | 0.050 | c3 | c4 |
| c(NO2)(mol/L) | 0.000 | 0.060 | c2 | 0.120 | 0.120 |
�ӱ��з�����c1________c2��c3________c4(�>������<������)��
(2)����ͼ�л����������˷�Ӧ��c(N2O4)��c(NO2)��Ũ����ʱ��仯�����ߡ�

(3)�����������£��ӷ�Ӧ��ʼֱ���ﵽ��ѧƽ��ʱ��������������ƽ����Ӧ����Ϊ__ mol/(L��s)��
(4)��Ӧ�ﵽƽ���,���������ĸı��ʹNO2�����Ũ���������
A�������������ݻ������� B���ٳ���һ������N2O4
C�������һ������NO2 D���ٳ���һ������He
��13�֣���(1)K�� �� ��< ������
�� ��< ������
(2)
(3)0.001��(4)B
 �� ��< ������
�� ��< ������(2)

(3)0.001��(4)B
����1��N2O4  2NO2
2NO2
��n: 0.10 0
20s: 0.07 0.06
40s 0.05 0.10
60s: 0.04 0.12
80s: 0.04 0.12
��3��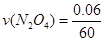 =0.001 mol/(L��s)
=0.001 mol/(L��s)
��4�������������ݻ���������Ũ�Ⱦ����٣���Ȼƽ���������ƣ���NO2�����Ũ�����ԭƽ����Ȼ�Ǽ�С���������������£����ݣ������һ������NO2����Ũ�ȼ��٣���Ȼƽ���������ƣ���NO2�����Ũ�����ԭƽ����Ȼ�Ǽ�С���ٳ���һ������He��������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ����ٳ���һ������N2O4��ƽ�������ƶ���NO2�����Ũ�����������⡣
 2NO2
2NO2��n: 0.10 0
20s: 0.07 0.06
40s 0.05 0.10
60s: 0.04 0.12
80s: 0.04 0.12
��3��
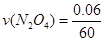 =0.001 mol/(L��s)
=0.001 mol/(L��s)��4�������������ݻ���������Ũ�Ⱦ����٣���Ȼƽ���������ƣ���NO2�����Ũ�����ԭƽ����Ȼ�Ǽ�С���������������£����ݣ������һ������NO2����Ũ�ȼ��٣���Ȼƽ���������ƣ���NO2�����Ũ�����ԭƽ����Ȼ�Ǽ�С���ٳ���һ������He��������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ����ٳ���һ������N2O4��ƽ�������ƶ���NO2�����Ũ�����������⡣

��ϰ��ϵ�д�
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�
�����Ŀ
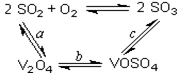
 SO3+ V2O4
SO3+ V2O4 
 2SO2+O2 ��550 ��ʱ��ƽ�ⳣ��K= ��
2SO2+O2 ��550 ��ʱ��ƽ�ⳣ��K= ��
 pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����:
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����: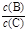 ��_________��
��_________�� 2NH3��g����H��0����ij����㶨���ܱ������а������1�s3����N2�� H2����һ�������·�����Ӧ����ͼ��ij��������Y����ʱ�䣨t���仯ʾ��ͼ��Y������(����)
2NH3��g����H��0����ij����㶨���ܱ������а������1�s3����N2�� H2����һ�������·�����Ӧ����ͼ��ij��������Y����ʱ�䣨t���仯ʾ��ͼ��Y������(����)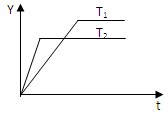
 CO(g)��Cl2(g)����H<0 �����й�˵����ȷ����(����)
CO(g)��Cl2(g)����H<0 �����й�˵����ȷ����(����)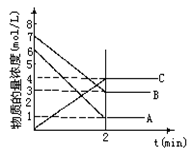
 4C
4C ���ﵽƽ��ʱ��C�����ʵ����ٷֺ���Ϊw����ά������������¶Ȳ��䣬���������ַ����ı���ʼ���ʣ��ﵽƽ���C�����ʵ����ٷֺ�������w����
���ﵽƽ��ʱ��C�����ʵ����ٷֺ���Ϊw����ά������������¶Ȳ��䣬���������ַ����ı���ʼ���ʣ��ﵽƽ���C�����ʵ����ٷֺ�������w����