��Ŀ����
����Ŀ����״���£�1.68L��ɫ�Ŀ�ȼ������������������ȫȼ�ա���������ͨ����������ʯ��ˮ�У��õ���ɫ��������Ϊ15g������������ʯ������ȼ�ղ������9.3g��
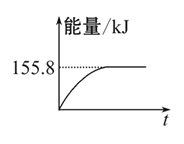
(1)ȼ�ղ�����ˮ������Ϊ_____g��
(2)��ԭ�����ǵ�һ���壬�������ʽΪ_____��������������Ƴɼ���(KOH)ȼ�ϵ�أ���д��������Ӧʽ_____��
(3)��ԭ�����������ֵ����ʵ�������̬����ɵĻ�����д�����ǵķ���ʽ________��(��д������)
(4)��ԭ�����������ֵ����ʵ�����������ɵĻ�������ֻ��һ����������д�����ǵķ���ʽ______��(��д������)
���𰸡�2.7 C2H4 C2H4-12e-+16OH- = 2![]() +10H2O CH4��C3H4��C2H2��C2H6 H2��C4H6��CO��C3H8
+10H2O CH4��C3H4��C2H2��C2H6 H2��C4H6��CO��C3H8
��������
�����л���ȼ��ʱ���л����е�̼ȫ��ת��Ϊ������̼��������̼������������������ȫ��Ӧ�����ɲ�����ˮ�İ�ɫ����̼��ƣ���������̼��Ƶ���������ȼ�����ɶ�����̼����������ʯ�Ҽ�������ȼ�����ɵ�ˮ����������ȼ�����ɵĶ�����̼����ˣ����ص�������ȼ�����ɶ�����̼��ˮ�������ܺͣ��Ӷ����ˮ���������������ɶ�����̼��ˮ�������������ȼ����C��HԪ�ص����ʵ������ٸ��������������ʽ���ݴ˽��
(1)��ȼ�ղ����ж�����̼������Ϊx��
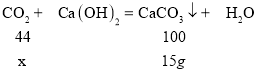 ��x=6.6g������ʯ�Ҽ�������ȼ�����ɵ�ˮ����������ȼ�����ɵĶ�����̼����ˣ����ص�������ȼ�����ɶ�����̼��ˮ�������ܺ�m(CO2)+m(H2O)=9.3g��m(H2O)=9.3g-6.6g=2.7g��
��x=6.6g������ʯ�Ҽ�������ȼ�����ɵ�ˮ����������ȼ�����ɵĶ�����̼����ˣ����ص�������ȼ�����ɶ�����̼��ˮ�������ܺ�m(CO2)+m(H2O)=9.3g��m(H2O)=9.3g-6.6g=2.7g��
(2)��ɫ��ȼ��������ʵ�����n=![]() = 0.075mol��n(CO2)=
= 0.075mol��n(CO2)=![]() =0.15mol����n(C)=0.15mol��n(H2O)=
=0.15mol����n(C)=0.15mol��n(H2O)=![]() =0.15mol����n(H)=n (H2O)��2=0.3mol����0.075mol�����к���0.15molC��0.3molH������n(����):n(C):n(H)= 0.075mol :0.15mol:0.3mol=1:2:4����1mol�����к���2molC��4molH�����Ը�����ķ���ʽ��C2H4��������������Ƴɼ���(KOH)ȼ�ϵ�أ���������������Ӧ��������ӦʽC2H4-12e-+16OH- = 2
=0.15mol����n(H)=n (H2O)��2=0.3mol����0.075mol�����к���0.15molC��0.3molH������n(����):n(C):n(H)= 0.075mol :0.15mol:0.3mol=1:2:4����1mol�����к���2molC��4molH�����Ը�����ķ���ʽ��C2H4��������������Ƴɼ���(KOH)ȼ�ϵ�أ���������������Ӧ��������ӦʽC2H4-12e-+16OH- = 2![]() +10H2O��
+10H2O��
(3)��Ϊ��һ����ΪC2H4����Ϊ�����ʵ����������������Ļ���������2mol��������У�Ӧ����4molCԭ�ӣ�8molHԭ�ӣ���������̬��������CH4��C3H4��C2H2��C2H6��
��4����Ϊ��һ����ΪC2H4����Ϊ�����ʵ�������������Ļ�������ֻ��һ��������������2mol��������У�Ӧ����4molCԭ�ӡ�8molHԭ�ӣ���������������ǣ�H2��C4H6 ��CO��C3H8�ȡ�