��Ŀ����
����Ŀ�������ӵ�����һ������������ӵ��������Ĺ������ʡ�ͼ1��Li3SBF4����ͼ2��DZ�ڵĿ����ӵ�����ϵĽṹʾ��ͼ���ش��������⣺
��1��S+2e-=S2-�����У���õĵ��������̬Sԭ�ӵ�___���������ţ���
��2��BF3+NH3=NH3BF3�ķ�Ӧ�����У��γɻ�ѧ��ʱ�ṩ���ӵ�ԭ�ӹ��������___����������в�ȡsp3�ӻ�����γɻ�ѧ����ԭ����___��
��3����̬Li+��B+�ֱ�ʧȥһ������ʱ�������ո�����������___��������___��
��4��ͼ1��ʾ�ľ����У��ԭ�Ӵ����������λ��___�����侧������Ϊapm�����ܶ�Ϊ___g��cm-3(�г�����ʽ����)
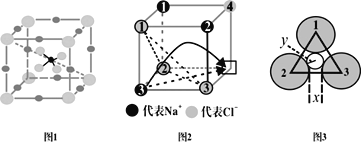
��5����ͼ2�з���������Na+ʱ��ǡ�ù����Ȼ��ƾ�����![]() �����Ȼ��ƾ�������a=564pm���Ȼ��ƾ����У�Cl-����A1�ܶѷ�ʽ�γɿ�϶��Na+�����������϶�С�ÿһ����϶��___��Cl-���ɣ���϶�Ŀռ���״Ϊ___��
�����Ȼ��ƾ�������a=564pm���Ȼ��ƾ����У�Cl-����A1�ܶѷ�ʽ�γɿ�϶��Na+�����������϶�С�ÿһ����϶��___��Cl-���ɣ���϶�Ŀռ���״Ϊ___��
��6���¶�����ʱ��NaCl�������ȱ�ݣ���ͼ2��ʾ��ijһ������û��Na+�����ֿ�λ��������ĵ����Դ����ǿ���þ��嵼��ʱ���ڵ糡������Ǩ�Ƶ���λ�ϣ��γɵ�����Ǩ�Ƶ�;������������ͼ2�м�ͷ��ʾ����
;��1����ƽ���ڼ���2��3��������֮������죨����Ϊx��Ǩ�Ƶ���λ��
;��2��������1��2��3���������γɵ�������ͨ������ͼ3��СԲ�İ뾶Ϊy��Ǩ�Ƶ���λ����֪��r(Na+)=95pm��r(Cl-)=185pm��![]() =1.4��
=1.4��![]() ��
��
��x=___��y=___��������һλС����
��Ǩ�ƿ����Ը����;����___��
���𰸡�3p 2p B��N Li+ Li+�����������Ų�Ϊ1s2��B+���������Ų�Ϊ2s2��1s����ϵĵ��ӵ���������2s����ϵ��� ���� ![]() 6 �������� 24.8pm 38.7pm ;��2
6 �������� 24.8pm 38.7pm ;��2
��������
��1������Sԭ�ӵĵ����Ų�ʽ��֪���չ�����õ��ĵ��������չ�����ɣ�
��2��B��N��2P���δ��������ӣ�
��3��Li+��1S���ʧȥ���ӡ�B+��2S���ʧȥ���ӣ�2S�����������1S�����
��4�����ݾ�̯�������ԭ�ӣ�����m=![]() v�����ܶȼ��ɣ�
v�����ܶȼ��ɣ�
��5���Ȼ��ƾ�����Na+�������������Խ����ϣ��ݴ˷�����
��6������ͼ��֪x=����������֮��ľ���-2r(Cl-),����������εĸߣ����������������ʿ���������ӵ�С�����ĵľ��룬�ټ�ȥ�����ӵİ뾶��Ϊyֵ��
�ڱȽ�x��yֵ���ɣ�˭��˭�����ܡ�
��1��Sԭ�ӵ����Ų�ʽΪ��1S22S22P63S23P4��֪��3P���δ��������ӣ��ʻ�õĵ��������̬Sԭ�ӵ�3P������ʴ�Ϊ��3P��
��2��H��B��N��F����ԭ�ӵĺ�����ӷ�����֪��N��Bԭ�Ӿ���δ�ɶԵ��ӣ������ṩ���ӵ�ԭ��ΪB��N��Bԭ�ӵĺ�������Ų�ʽΪ��1S22S22P1,Nԭ�ӵĺ�������Ų�ʽΪ��1S22S22P3������Bԭ�ӵ�2P�����Nԭ�ӵ�2P����ṩ���ӣ���B��Nԭ�Ӻ�������Ų���֪B��Nԭ�Ӿ��ṩ3��2P���������sp3�ӻ���ΪNԭ�ӣ��ʴ�Ϊ��2P��B��N��
��3��Li+��1S���ʧȥ���ӡ�B+��2S���ʧȥ���ӣ�2S�����������1S�������1S���ʧȥ�������ո�����������ʴ�Ϊ��Li+ ��Li+�������������Ų�Ϊ1s2��B+���������Ų�Ϊ2s2��1s����ϵĵ��ӵ���������2s����ϵ��ӣ�
��4�����ϵ�ԭ�Ӹ���=12��![]() =3�������ϵ�ԭ�Ӹ���=8��
=3�������ϵ�ԭ�Ӹ���=8��![]() =1�����ĵ�ԭ�Ӹ���=1���������ڲ�������ԭ�Ӹ���=4����Li3SBF4��֪���þ�����������ﮣ������ԭ�Ӹ��ݾ�̯�������ͼƬ��ͼ���ԭ�Ӹ���
=1�����ĵ�ԭ�Ӹ���=1���������ڲ�������ԭ�Ӹ���=4����Li3SBF4��֪���þ�����������ﮣ������ԭ�Ӹ��ݾ�̯�������ͼƬ��ͼ���ԭ�Ӹ���![]() 4=3�����������λ�����ϣ�1mol����������=(7��3+32��1+11��1+19��4)g140g����Ϊ��������Ϊa pm=a��10-10cm��һ�����������=a3=a3��10-30cm3��1mol���������= a3��10-30cm3��NA����m=
4=3�����������λ�����ϣ�1mol����������=(7��3+32��1+11��1+19��4)g140g����Ϊ��������Ϊa pm=a��10-10cm��һ�����������=a3=a3��10-30cm3��1mol���������= a3��10-30cm3��NA����m=![]() v�ã�
v�ã�![]() =
=![]() g��cm-3���ʴ�Ϊ�����ϣ�
g��cm-3���ʴ�Ϊ�����ϣ�![]() ��
��
��5���Ȼ��ƾ����У�Cl-����A1�ܶѣ�ÿ��Cl-��Χ��6��Na+,ÿ��Na��Χ��6��Cl-���ռ���״Ϊ�������壬�ʴ�Ϊ��6���������壻
��6������ͼ2ΪNaCl����İ˷�֮һ���壬����ͼ2��С�������ⳤ=![]() =
=![]() =282pm��������Cl-֮��ľ���=
=282pm��������Cl-֮��ľ���=![]() ��282pm��
��282pm��
����ͼ��֪x=����������֮��ľ���-2r(Cl-)=![]() ��282pm-2��185pm=24.8pm����ͼ��֪�������εĸ�cos30�������������֮��ľ���=
��282pm-2��185pm=24.8pm����ͼ��֪�������εĸ�cos30�������������֮��ľ���=![]() ���������������ʿ�֪��������С�����ĵľ���=
���������������ʿ�֪��������С�����ĵľ���=![]() �������εĸ�=
�������εĸ�=![]()
![]() ����ͼ��֪y=�����ӵ�С�����ĵľ���-r(Cl-)=
����ͼ��֪y=�����ӵ�С�����ĵľ���-r(Cl-)=![]()
![]() -185pm=38.72pm,����һλС��Ϊ38.7pm���ʴ�Ϊ��24.8pm��38.7pm��
-185pm=38.72pm,����һλС��Ϊ38.7pm���ʴ�Ϊ��24.8pm��38.7pm��
����Ϊx<y���ڶ��ּ�϶�������Եڶ���;�������ܣ��ʴ�Ϊ��;��2��

����Ŀ�����Ż�����ʶ��ǿ�������ԴԽ��Խ�ܵ����ǹ�ע��
��1��������һ������Ľྻȼ�ϡ���֪��CH4��g��+2O2��g��= CO2��g��+ 2H2O��g������H= -802.3kJ��mol-1 H2O��1��=H2O��g������H =+44.0kJ��mol-l
д�����³�ѹ�¼�����ȫȼ�յ��Ȼ�ѧ����ʽ____������4.8g����������ȫȼ������Һ̬ˮ���ų�����Ϊ____kJ��
��2�����ü�����ˮ��Ӧ�Ʊ���������ԭ�����ۣ������ƹ��ֵ���÷�ӦΪCH4��g��+H2O��g��![]() CO��g��+3H2��g����H=+206.lkJ��mol-l��
CO��g��+3H2��g����H=+206.lkJ��mol-l��
��800��ʱ����Ӧ�Ļ�ѧƽ�ⳣ��K=l.0��ijʱ�̲�ø��¶����ܱ������и����ʵ����ʵ���Ũ�����±���
CH4 | H2O | CO | H2 |
3.0 molL1 | 8.5 molL1 | 2.0 molL1 | 2.0 molL1 |
���ʱ�����淴Ӧ���ʵĹ�ϵ������Ӧ����____�淴Ӧ���ʡ��������������������������
��Ϊ��̽���¶ȡ�ѹǿ��������ѧ��Ӧ���ʵ�Ӱ�죬ijͬѧ�������������Ա�ʵ�飨�¶�Ϊ360���480�桢ѹǿΪ101 kPa��303 kPa������ʵ���������±�����
ʵ����� | �¶�/�� | ѹǿ/kPa | CH4��ʼŨ��/ molL1 | H2O��ʼŨ��/ molL1 |
1 | 360 | p | 2.00 | 6.80 |
2 | t | 101 | 2.00 | 6.80 |
3 | 360 | 101 | 2.00 | 6.80 |
����t=___��P=____�����ʵ��2��3��Ŀ����____��ʵ��l��2��3�з�Ӧ�Ļ�ѧƽ�ⳣ���Ĵ�С��ϵ��____����K1��K2��K3��ʾ����