��Ŀ����
1��2SO2��g��+O2��g��?2SO3��g���ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ĸ����������Ϊ2L�ĺ����ܱ�������Ͷ�ϣ�����ʼ���ʵ���������Ӧ���ƽ��ת�������±���ʾ��| �� | �� | �� | �� | ||
| ��ʼ���ʵ��� | n��SO2��/mol | 0.40 | 0 | 0.80 | 0.02 |
| n��O2��/mol | 0.24 | 0 | 0.48 | 0.04 | |
| n��SO3��/mol | 0 | 0.40 | 0 | 0.40 | |
| ����Ӧ���ƽ��ת����% | 80 | a1 | a2 | a3 | |
| A�� | ���¶��£�ƽ�ⳣ����ֵΪ400 | B�� | ƽ��ʱ������c��SO3���Ǽ��е�2�� | ||
| C�� | ƽ��ʱ��a3��a1 | D�� | ����SO3��ƽ��ת����Ϊa1=20% |
���� A������ƽ��ʱ�����������ת����Ϊ80%����ת���Ķ�������Ϊ0.4mol��80%=0.32mol����
2SO2��g��+O2��g��?2SO3��g��
��ʼ����mol����0.4 0.24 0
�仯����mol����0.32 0.16 0.32
ƽ������mol����0.08 0.08 0.32
�ٸ���ƽ�ⳣ������ʽK=$\frac{{c}^{2}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$���㣻
B������ЧΪ�ڼ�ƽ��Ļ�����ѹǿ����һ����ƽ�������ƶ������ж��������ת��������
C������ЧΪ����ƽ��Ļ������ڼ���0.02molSO2��0.04molO2��ƽ�������ƶ������ж�������ת���ʼ�С��
D����ʼͶ��0.4molSO3����ʼͶ��0.4molSO2��0.2molO2����ȫ��Чƽ�⣬�ҵ�ЧΪ�ڼ���ƽ��Ļ�����������0.04molO2������ƽ��ʱ������������ʵ���С��0.32mol�������зֽ�������������0.4mol-0.32mol=0.08mol��
��� �⣺A������ƽ��ʱ�����������ת����Ϊ80%����ת���Ķ�������Ϊ0.4mol��80%=0.32mol����
2SO2��g��+O2��g��?2SO3��g��
��ʼ����mol����0.4 0.24 0
�仯����mol����0.32 0.16 0.32
ƽ������mol����0.08 0.08 0.32
ƽ�ⳣ��K=$\frac{{c}^{2}��S{O}_{3}��}{{c}^{2}��S{O}_{2}����c��{O}_{2}��}$=$\frac{��\frac{0.32}{2}��^{2}}{��\frac{0.08}{2}��^{2}��\frac{0.08}{2}}$=400����A��ȷ��
B������ЧΪ�ڼ�ƽ��Ļ�����ѹǿ����һ����ƽ�������ƶ������ж��������ת��������ƽ��ʱ����c��SO3�����ڼ��е�2������B����
C������ЧΪ����ƽ��Ļ������ڼ���0.02molSO2��0.04molO2��ƽ�������ƶ������ж�������ת���ʼ�С����ƽ��ʱa3��a1����C����
D����ʼͶ��0.4molSO3����ʼͶ��0.4molSO2��0.2molO2����ȫ��Чƽ�⣬�ҵ�ЧΪ�ڼ���ƽ��Ļ�����������0.04molO2������ƽ��ʱ������������ʵ���С��0.32mol�������зֽ�������������0.4mol-0.32mol=0.08mol���������������ת����a1��$\frac{0.08mol}{0.4mol}$��100%=20%����D����
��ѡ��A��
���� ���⿼�黯ѧƽ���йؼ��㡢ƽ�ⳣ�����㡢��Чƽ��ȣ��Ѷ��еȣ�Dѡ��Ϊ�ѵ㣬ѧ����������ƽ�ⳣ��������BCD��ע�����õ�Ч˼�빹��ƽ�⽨����;����������ƽ���ƶ��������

 �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |

��д���пհף�
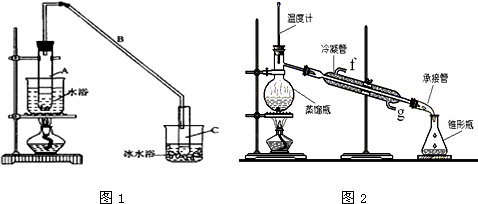
��1����Ӧ����A����μ�����ͱ��Ļ��Һ���������ھͷ�����Ӧ��д��A����������Ӧ�Ļ�ѧ����ʽ���л���д�ṹ��ʽ����
 ��
����2���Թ�C�б��������dz�ȥ�廯���л��е�����������Ӧ��ʼ�۲�D�Թܣ�����������Ϊʯ����Һ��죬���ڵ������а�������
��3������������װ���У����з��������õ�������DEF������ĸ����
��4����Ӧ��Ϻ�aƿ�е�Һ��ȡ����������Һ ���������������ƣ��ɻ�û�ô��屽������屽�м�����ˮCaCl2����Ŀ���Ǹ���
��5�������Ϸ��������Ҫ��һ���ᴿ���屽�����в����б������C��������ȷѡ��ǰ����ĸ����
A���ؽᾧ����B�����ˡ���C������D����ȡ��
�ٱ�����Һ���ȱ����
�ڱ�����Һ�м�NaOH����Һ����壬���ɱ����ƺ�ˮ
�۱��ӿ���FeCl3��Ӧ
���ڱ�����Һ�м���Ũ��ˮ������ɫ����
�ݱ�������Һ��ͨ��CO2�����ֻ���
�ޱ�������Na2CO3��Һ��Ӧ�ų�CO2��
| A�� | �ڢ� | B�� | �� | C�� | �ۢܢ� | D�� | �ۢܢ� |
��1�������£�ij��ˮ��pH=12����ˮ�����c��OH-��=1��10-12mol/L������ð�ˮ�м��������������ʵ���Ũ�ȵ����ᣬ��ʱ��Һ��ˮ����ij̶ȴ��ڣ�����ڡ��������ڡ���С�ڡ�����ˮ��ˮ�ĵ���̶ȣ�
��2���ϳɰ���ӦN2��g��+3H2��g��?2NH3��g�������ں��¡���ѹ��������ƽ����ϵ��ͨ���������ƽ�������ƶ����������Ӧ���������淴Ӧ����������
��3��һ���¶��£����ܱ������пɷ������з�Ӧ��2N2O5��g��?4NO2��g��+O2��g�����±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ���500s��N2O5�ķֽ�����v��N2O5��=3��10-3mol•L-1•s-1��������T2����Ӧ����l000sʱ���c��NO2��=4.98mol•L-1����Ӧ2N2O5��g��?4NO2��g��+O2��g���ġ�H��0�������������=����������
| t���룩 | 0 | 500 | 1000 |
| N2O5Ũ��mol•L-1 | 5.00 | 3.50 | 2.42 |
| A�� | HCl | B�� | Na | C�� | Na2SO4 | D�� | NaHSO4 |
 ������Һ����ʹú��������Դ����Ч;����ú����������Ҫ��Ӧ��C+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO+H2��CO��H2�Ļ�������Ǻϳɶ����л����ԭ�������о���CO��H2�ϳ��л���Ļ�ѧ��Ϊһ̼��ѧ����ͼ�Ǻϳ�ijЩ���ʵ�·��ͼ��
������Һ����ʹú��������Դ����Ч;����ú����������Ҫ��Ӧ��C+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO+H2��CO��H2�Ļ�������Ǻϳɶ����л����ԭ�������о���CO��H2�ϳ��л���Ļ�ѧ��Ϊһ̼��ѧ����ͼ�Ǻϳ�ijЩ���ʵ�·��ͼ��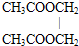 +2H2O����A��B��2CH3OH+O2$��_{��}^{Cu}$2HCHO+2H2O��
+2H2O����A��B��2CH3OH+O2$��_{��}^{Cu}$2HCHO+2H2O��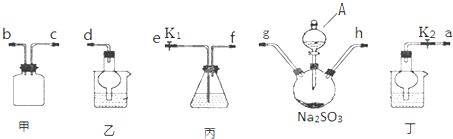
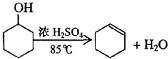
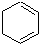 ������д����������Ӧ��Ӧ�Ļ�ѧ����
������д����������Ӧ��Ӧ�Ļ�ѧ����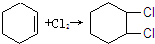 ��
�� ��
��