��Ŀ����
6����ҵ�ϳ����ú����ˮ����Na2S2O3•5H2O��ʵ���ҿ�������װ�ã���ȥ���ּӳ�������ģ�����ɹ��̣�
��ƿC�з�����Ӧ���£�
Na2S��aq��+H2O��l��+SO2��g���TNa2SO3��aq��+H2S��aq�� ����
2H2S��aq��+SO2��g���T3S��s��+2H2O��l�� ����
S��s��+Na2SO3��aq��$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3��aq�� ����
��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һע����Һ���߶ȱ��ֲ��䣬������װ�����������ã�װ��D�������Ƿ�ֹ������װ��E��ΪNaOH��Һ��
��2��Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ2��1��
��3��װ��B������֮һ�ǹ۲�SO2���������ʣ� ʵ���У�ΪʹSO2����������ƿC�����õIJ����ǿ��Ƶμ���������ʣ���֪��Ӧ������Խ���������ƿC�з�Ӧ�ﵽ�յ����������Һ����壨�������ʧ������Ӧ���ڿ��þƾ����ʵ�������ƿA��ʵ�����þƾ��Ƽ���ʱ����ʹ��ʯ��������������ad��
a���ձ� b�������� c���Թ� d����ƿ
��4����Ӧ��ֹ����ƿC�е���Һ������Ũ����������Na2S2O3•5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʣ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�ȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
����֪Na2S2O3•5H2O�����ֽ⣺S2O32?+2H+�TS��+SO2��+H2O
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ��
���� ��ҵ�ϳ����ú����ˮ����Na2S2O3•5H2O������װ��A�Ʊ��������ѷ�Һ©���е�Ũ���������ƿ�����������з�Ӧ�õ������������壬��������ͨ��Bװ�ã�װ��B������֮һ�ǹ۲�SO2���������ʣ� ʵ����ΪʹSO2����������ƿC����Ҫ���ƿ��Ƶμ���������ʣ�ͨ��װ��C������Ӧ��ƿC�з�����Ӧ���£�
Na2S��aq��+H2O��l��+SO2��g���TNa2SO3��aq��+H2S��aq�� ����
2H2S��aq��+SO2��g���T3S��s��+2H2O��l�� ����
S��s��+Na2SO3��aq��$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3��aq�� ����
��Ӧ��ֹ����ƿC�е���Һ������Ũ����������Na2S2O3•5H2O���õ���������ƣ�װ��D�Ƿ�ֹ�����İ�ȫƿ��װ��E������ʣ�����壬��ֹ��Ⱦ������
��1������Һ�����һ��ʱ�䲻����������ԣ�D�ɷ�ֹҺ�嵹����E��ʢ��NaOH��Һ����β��������
��2��C��Na2S��Na2SO3ǡ����ȫ��Ӧ������ձ�C�еķ�Ӧ������
��3��װ��B������֮һ�ǹ۲�SO2���������ʣ����ƶ������������������ȣ�ʹ�����ֻ�ϣ�ΪʹSO2����������ƿC��Ӧʹ������������������������Դӵ���������ʿ��ƣ���ƿC�з�Ӧ�ﵽ�յ�˵��ǡ�÷�Ӧ������������ƣ���Һ����壬����������������ϴ��������Ҫ���ȼ��ȣ�����ֲ�����ը�Ѳ���������
��4������Ʒ���Ƿ����Na2SO4���ȼ������ų����ţ��������Ȼ����������������
��� �⣺��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һ������Һ���߶ȱ��ֲ��䣬�����������ã�D�����Ϊ�̵��ܣ�Ϊ��ȫƿ����ֹ������װ��E������β����SO2��H2S�����ã���ѡ��NaOH��Һ��
�ʴ�Ϊ��Һ���߶ȱ��ֲ��䣬��ֹ������NaOH��
��2��C��Na2S��Na2SO3ǡ����ȫ��Ӧ����Na2S��aq��+H2O��l��+SO2��g���TNa2SO3��aq��+H2S��aq������
2H2S��aq��+SO2��g���T3S��s��+2H2O��l������
S��g��+Na2SO3��aq��$\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3��aq������
��֪������2+����+����3���õ��ܷ�ӦΪ2Na2S��aq��+Na2SO3��aq��+3SO2��g��$\frac{\underline{\;\;��\;\;}}{\;}$3Na2S2O3��aq������C��Na2S��Na2SO3���ʵ���֮��Ϊ2��1��
�ʴ�Ϊ��2��1��
��3��װ��B������֮һ�ǹ۲�SO2���������ʣ����ƶ������������������ȣ�ʹ�����ֻ�ϣ�ΪʹSO2����������ƿC��Ӧʹ������������������������Դӵ���������ʿ��ƣ�����C�з����ķ�Ӧ��֪����ƿC�з�Ӧ�ﵽ�յ㷢����ӦΪ�����������Ʒ�Ӧ������������ƣ�����Ӧ������Ϊ��Һ����壨�������ʧ��������������������ϴ��������Ҫ���ȼ��ȣ�����ֲ�����ը�Ѳ����������ձ�����ƿ������Ҫ��ʯ�������Թ�������ֱ�Ӽ��ȣ�ѡad��
�ʴ�Ϊ�����Ƶμ�������ٶȣ���Һ����壨�������ʧ����ad��
��4������Ʒ���Ƿ����Na2SO4������������ͽ���Ϊȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
�ʴ�Ϊ��ȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
���� ���⿼��ʵ�鷽���ķ��������ۣ��漰�����Լ��顢���Ӽ��顢�Բ����ķ������ۡ���ѧ����ȣ�����ʵ�����������֪ʶ�ۺ�Ӧ�������Ŀ��飬���������ܷ�Ӧ�ķ�������Ŀ�Ѷ��еȣ�

| A�� | Cu2O+H2SO4��CuSO4+Cu+H2O | B�� | FeO+HNO3��Fe��NO3��3+H2O | ||
| C�� | NH4NO3��N2+H2O | D�� | S+KOH��K2SO3+K2S+H2O |
| �� | �� | �� | �� | ||
| ��ʼ���ʵ��� | n��SO2��/mol | 0.40 | 0 | 0.80 | 0.02 |
| n��O2��/mol | 0.24 | 0 | 0.48 | 0.04 | |
| n��SO3��/mol | 0 | 0.40 | 0 | 0.40 | |
| ����Ӧ���ƽ��ת����% | 80 | a1 | a2 | a3 | |
| A�� | ���¶��£�ƽ�ⳣ����ֵΪ400 | B�� | ƽ��ʱ������c��SO3���Ǽ��е�2�� | ||
| C�� | ƽ��ʱ��a3��a1 | D�� | ����SO3��ƽ��ת����Ϊa1=20% |
| AX | BX | AY | BY |
| pH=7��c��X-��=1mol/L | pH=4 | pH=6 |
��2������Ũ�ȵ�HX��HYϡ����ͬ�ı�������Һ��pH�仯���ȣ�HX��HY���������������pH��AOH��BOH��Һ��ϡ��ʱpH���½�2�����ˮ����AOH��BOH�������������������=������
��3����BX��Һ�У�c��B+��+c��H+��-c��OH-��=1mol/L��
��AY��Һ�У�c��A+��-c��Y-��=c��HY������һ���ʾ��=c��OH-��-c��H+�����������ʾ����
��4��A2Z��ˮ�ⷽ��ʽ��Z2-+H2O?HZ-+OH-��HZ-+H2O?H2Z+OH-��
 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Kal=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��������Һ��a��CH3COONa b��Na2CO3 c��NaClO
pH��С�������е�˳����a��c��b���ñ����д����
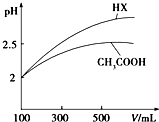
��2�������Ϊ100mL pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ�����С�ڡ����ڡ���CH3COOH�ĵ���ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������C��H+�����ڣ�����ڡ�����С�ڡ����ڡ���������Һ��ˮ���������C��H+����
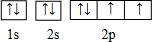 ������ӵ�����������2�֣��Ƚ�����ͬ����ǽ���Ԫ���γɵĵ��ʵ������ԣ��û�ѧ����ʽ��ʾ��2H2S+O2=2S��+2H2O
������ӵ�����������2�֣��Ƚ�����ͬ����ǽ���Ԫ���γɵĵ��ʵ������ԣ��û�ѧ����ʽ��ʾ��2H2S+O2=2S��+2H2O ����Ҫ��Br-��ʽ�����ں�ˮ�У���ˮ�������ԣ���ҵ���Ʊ���Br2�IJ�������Ϊ��
����Ҫ��Br-��ʽ�����ں�ˮ�У���ˮ�������ԣ���ҵ���Ʊ���Br2�IJ�������Ϊ��