ЬтФПФкШн
IЃЎДПМюЁЂЩеМюЕШЪЧживЊЕФЛЏЙЄдСЯЁЃ

ЃЈ1ЃЉРћгУЩЯЭМЫљЪОзАжУПЩМфНгжЄУїЖўбѕЛЏЬМгыЩеМюШмвКЗЂЩњСЫЗДгІЁЃНЋAгыBСЌНгЃЌДђПЊжЙЫЎМаЃЌНЋНКЭЗЕЮЙмжаЕФвКЬхМЗШыЩеЦПЃЌДЫЪБЕФЪЕбщЯжЯѓЪЧ___________________ЃЌ
ШєЦфЫћВйзїВЛБфЃЌНЋAгыCСЌНгЃЌПЩЙлВьЕНЕФЯжЯѓЪЧ__________________________ЁЃ
ЃЈ2ЃЉЯђNaOHШмвКжаЭЈШывЛЖЈСПCO2ЃЌНсОЇКѓЕУЕНАзЩЋЙЬЬхЃЌИУАзЩЋЙЬЬхЕФзщГЩПЩФмЪЧЃК
AЃЎNaOHКЭNa2CO3ЃЛBЃЎЁЁЁЁЁЁЁЁЁЁЁЁЃЛCЃЎЁЁЁЁЁЁЁЁЁЁЁЁЁЁЃЛDЃЎЁЁЁЁЁЁЁЁЁЁЁЁЁЃ
ЃЈ3ЃЉЩшМЦЪЕбщШЗЖЈЃЈ2ЃЉжаАзЩЋЙЬЬхжаДцдкAЯюжаЕФвѕРызгЃК
ЪЕбщВйзї | ЪЕбщЯжЯѓ | НсТл |
ЂйШЁЩйСПАзЩЋЙЬЬхгкЪдЙмжаЃЌМгзуСПЫЎШмНтЃЌдйМгзуСПBaCl2ШмвК |
|
|
Ђк
|
|
|
IIЃЎЛЏбЇаЫШЄаЁзщЖдФГЦЗХЦбРИржаЕФФІВСМСГЩЗжМАЦфКЌСПНјаавдЯТЬНОПЃК
ВщЕУзЪСЯЃКИУбРИрФІВСМСгЩЬМЫсИЦЃЌЧтбѕЛЏТСзщГЩЃЛбРИржаЦфЫћГЩЗжгіЕНбЮЫсЪБЮоЦјЬхВњЩњЁЃ
бРИрбљЦЗжаЬМЫсИЦЕФЖЈСПВтЖЈЃКРћгУЯТЭМЫљЪОзАжУ(ЭМжаМаГжвЧЦїТдШЅ)НјааЪЕбщЃЌГфЗжЗДгІ
КѓЃЌВтЖЈCжаЩњГЩЕФBaCO3ГСЕэжЪСПЃЌвдШЗЖЈЬМЫсИЦЕФжЪСПЗжЪ§ЁЃ

вРОнЪЕбщЙ§ГЬЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЪЕбщЙ§ГЬжаашГжајЛКЛКЭЈШыПеЦјЁЃЦфзїгУГ§СЫПЩНСАшBЃЌCжаЕФЗДгІЮяЭтЃЌЛЙгаЃК ЁЃ
ЃЈ2ЃЉCжаЗДгІЩњГЩBaCO3ЕФРызгЗНГЬЪНЪЧ ЁЃ
ЃЈ3ЃЉЯТСаИїЯюДыЪЉжаЃЌВЛФмЬсИпВтЖЈзМШЗЖШЕФЪЧЃЈ ЃЉ
AЃЎдкМгШыбЮЫсжЎЧАЃЌгІХХОЛзАжУФкЕФCO2ЦјЬх
BЃЎЕЮМгбЮЫсВЛвЫЙ§Пь
CЃЎдкAЁЋBжЎМфдіЬэЪЂгаХЈСђЫсЕФЯДЦјзАжУ
DЃЎдкBЁЋCжЎМфдіЬэЪЂгаБЅКЭЬМЫсЧтФЦШмвКЕФЯДЦјзАжУ
ЃЈ4ЃЉЪЕбщжазМШЗГЦШЁ8.00 gбљЦЗШ§ЗнЃЌНјааШ§ДЮВтЖЈЃЌВтЕУBaCO3ЦНОљжЪСПЮЊ3.94 g.дђбљЦЗжаЬМЫсИЦЕФжЪСПЗжЪ§ЮЊ________ЁЃ
ЃЈ5ЃЉгаШЫШЯЮЊВЛБиВтЖЈCжаЩњГЩЕФBaCO3жЪСПЃЌжЛвЊВтЖЈзАжУCдкЮќЪеCO2ЧАКѓЕФжЪСПВюЃЌвЛбљПЩвдШЗЖЈЬМЫсИЦЕФжЪСПЗжЪ§ЁЃЪЕбщжЄУїАДДЫЗНЗЈВтЖЈЕФНсЙћУїЯдЦЋИпЃЌдвђЪЧ _________________________________ЁЃ
ЃЈ6ЃЉзАжУжаUаЮЙмDжаЕФМюЪЏЛвЕФзїгУЪЧ_____________________________ЁЃ
IЃЎЃЈ1ЃЉЫЎбиЕМЙмгЩЙуПкЦПНјШыЩеЦП ЙуПкЦПжаЕФГЄЕМЙмПкгаЦјХнВњЩњ
ЃЈ2ЃЉNa2CO3 Na2CO3КЭNaHCO3 NaHCO3
ЃЈ3ЃЉ
ЪЕбщВйзї | ЪЕбщЯжЯѓ | НсТл |
ЃЈ1ЃЉ | ВњЩњАзЩЋГСЕэ | гаCO32Ѓ |
ЃЈ2ЃЉЙ§ТЫЃЌШЁЩйСПТЫвКгкЪдЙмжаЃЌЕЮМгЗгЬЊ | ТЫвКБфКь | гаOHЃ |
IIЃЎЃЈ1ЃЉАбЩњГЩЕФCO2ШЋВПХХШыCжаЃЌЪЙжЎЭъШЋБЛBa(OH)2ШмвКЮќЪе
ЃЈ2ЃЉBa2++2OH-+CO2=BaCO3Ё§+H2O ЃЈ3ЃЉC D ЃЈ4ЃЉ25%
ЃЈ5ЃЉBжаЫЎеєЦјЃЌТШЛЏЧтЦјЬхЕШНјШыCзАжУжа ЃЈ6ЃЉЗРжЙПеЦјжаЕФCO2НјШыC
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКIЃЎЃЈ1ЃЉCO2+2NaOH=Na2CO3+H2OгЩгкЖўепЗЂЩњЗДгІЕМжТAзАжУЕФЦјЬхбЙЧПМѕаЁЃЌBзАжУжаЕФЫЎдкДѓЦјбЙЕФзїгУЯТОЕМЦјЙмНјШыAзАжУЁЃШєЦфЫћВйзїВЛБфЃЌНЋAгыCСЌНгЃЌПЩЙлВьЕНЕФЯжЯѓЪЧЙуПкЦПжаЕФГЄЕМЙмПкУАЦјХнЁЃЃЈ2ЃЉЯђNaOHШмвКжаЭЈШывЛЖЈСПCO2ЃЌПЩФмЗЂЩњЕФЗДгІЮЊЃК2NaOH+CO2= Na2CO3+H2OЃЌNaOH+CO2= NaHCO3ЁЃЫљвдНсОЇКѓЕУЕНАзЩЋЙЬЬхЃЌИУАзЩЋЙЬЬхЕФзщГЩПЩФмЪЧЃКAЃЎNaOHКЭNa2CO3ЃЛBЃЎNa2CO3ЃЛCЃЎNa2CO3КЭNaHCO3ЃЛDЃЎNaHCO3ЁЃЃЈ3ЃЉЂйШЁЩйСПАзЩЋЙЬЬхгкЪдЙмжаЃЌМгзуСПЫЎШмНтЃЌдйМгзуСПBaCl2ШмвКЃЌВњЩњАзЩЋГСЕэжЄУїКЌгаCO32ЃРызгЁЃЂкЙ§ТЫЃЌШЁЩйСПТЫвКгкЪдЙмжаЃЌЕЮМгЗгЬЊЃЌТЫвКБфКьжЄУїКЌгаOHЃЁЃ
IIЃЎЃЈ1ЃЉЪЕбщЙ§ГЬжаашГжајЛКЛКЭЈШыПеЦјЁЃЦфзїгУГ§СЫПЩНСАшBЃЌCжаЕФЗДгІЮяЭтЃЌЛЙгаАбЩњГЩЕФCO2ШЋВПХХШыCжаЃЌЪЙжЎЭъШЋБЛBa(OH)2ШмвКЮќЪеЁЃЃЈ2ЃЉCжаЗДгІЩњГЩBaCO3ЕФРызгЗНГЬЪНЪЧBa2++2OH-+ CO2= BaCO3Ё§+H2OЁЃЃЈ3ЃЉAЃЎдкМгШыбЮЫсжЎЧАЃЌгІХХОЛзАжУФкЕФCO2ЦјЬхПЩвдМѕЩйПеЦјжаЕФЖўбѕЛЏЬМЖдВњЩњГСЕэЕФгАЯьЃЌМѕаЁЪЕбщЮѓВюЃЌЬсИпЪЕбщЕФзМШЗЖШЁЃДэЮѓЁЃBЃЎЕЮМгбЮЫсВЛвЫЙ§ПьПЩвдЪЧЗДгІВњЩњЕФЖўбѕЛЏЬМЦјЬхБЛЧтбѕЛЏБЕШмвКГфЗжЮќЪеЃЌЬсИпЗДгІЕФзМШЗЖШЁЃДэЮѓЁЃCЃЎдкAЁЋBжЎМфдіЬэЪЂгаХЈСђЫсЕФЯДЦјзАжУЃЌЮќЪеЫЎЗжЖдЖўбѕЛЏЬМКЌСПЕФВтЖЈЮоШЛКѓгАЯьЁЃе§ШЗЁЃDЃЎШєдкBЁЋCжЎМфдіЬэЪЂгаБЅКЭЬМЫсЧтФЦШмвКЕФЯДЦјзАжУЃЌдђгЩЗДгІЮяЛгЗЂГіРДЕФHClдгжЪгжгыЦфЗДгІВњЩњЖўбѕЛЏЬМЦјЬхЃЌЪЙВтЖЈНсЙћЦЋДѓЁЃе§ШЗЁЃЙЪбЁЯюЮЊЃКC DЁЃЃЈ4ЃЉгЩЗНГЬЪНCaCO3+2HCl=CaCl2+ CO2Ёќ+H2O; CO2+ Ba(OH)2= BaCO3Ё§+H2OЁЃПЩЕУЙиЯЕЪНCaCO3-- BaCO3ЁЃМД100g CaCO3ЁЊ197g BaCO3.НёBaCO3жЪСПЮЊ3.94 gЃЌЫљвдКЌгаЕФCaCO3ЕФжЪСПЮЊ2.0g. бљЦЗжаЬМЫсИЦЕФжЪСПЗжЪ§ЮЊ(2.0gЁТ8.0g)ЁС100%=25%.ЃЈ5ЃЉBжаЫЎеєЦјЃЌТШЛЏЧтЦјЬхЕШНјШыCзАжУжа.ЃЈ6ЃЉзАжУжаUаЮЙмDжаЕФМюЪЏЛвЕФзїгУЪЧЗРжЙПеЦјжаЕФCO2НјШыC ,вдЗРжЙгАЯьЪЕбщВтЖЈНсЙћЕФзМШЗадЁЃ
ПМЕуЃКПМВщЖўбѕЛЏЬМЕФЛЏбЇаджЪМАдкЪЕбщЙ§ГЬжаЮяжЪГЩЗжЕФВтЖЈЁЂдгжЪЕФГ§ШЅЁЂМАПЩФмВњЩњЪЕбщЮѓВюЕФЗжЮіЕШжЊЪЖЁЃ

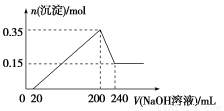
ШЁ20 mL NaOHШмвКЦНОљЗжГЩСНЗнЃЌЗжБ№ЗХШыAЁЂBСНжЇЪдЙмжаЁЃЯђAЁЂBжаЭЈШыВЛЕШСПЕФCO2ЃЌдйМЬајЯђСНШмвКжаж№ЕЮМгШы0.1mol/LЕФбЮЫсЃЌБъзМзДПіЯТВњЩњЕФCO2ЦјЬхЬхЛ§гыЫљМгЕФбЮЫсШмвКЬхЛ§жЎМфЕФЙиЯЕШчЯТБэЫљЪОЃК
бЮЫсЬхЛ§ЃЈЕЅЮЛЃКmLЃЉ | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
AВњЩњCO2ЕФЬхЛ§ | 0 | 0 | 0 | 0 | 0 | 22.4 | 44.8 | 44.8 | 44.8 |
BВњЩњCO2ЕФЬхЛ§ | 0 | 0 | 22.4 | 44.8 | 67.2 | 89.6 | x | x | x |
ЧыЛиД№ЯТСаЮЪЬт
ЃЈ1ЃЉЩйСПCO2гыNaOHШмвКЗДгІЕФРызгЗНГЬЪН ЃЌ
Й§СПCO2гыNaOHШмвКЗДгІЕФЛЏбЇЗНГЬЪН ЃЛ
ЃЈ2ЃЉЪдЙмAжаЭЈШыCO2КѓЫљЕУШмвКЕФШмжЪЮЊ ЃЛ
ЃЈ3ЃЉдNaOHШмвКЕФЮяжЪЕФСПХЈЖШЮЊ mol/LЃЛ
ЃЈ4ЃЉЕЮМг70mLбЮЫсЪБЃЌAЁЂBВњЩњCO2ЕФЬхЛ§ОљЮЊзюДѓжЕЃЌдђx= mLЁЃ