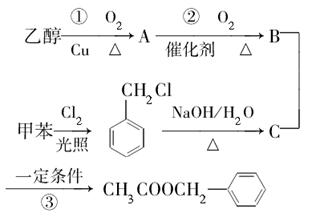
��Ŀ����
(18��)������I(C11H12O3)���Ʊ�Һ�����ϵ��м���֮һ��������к���ȩ����������I������E��H��һ�������ºϳɣ�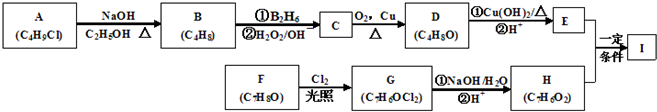
��֪������Ϣ���� A�ĺ˴Ź������ױ�����ֻ��һ�ֻ�ѧ�������⣻
��R��CH��CH2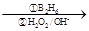 R��CH2CH2OH���ۻ�����F�����ϵ�һ�ȴ���ֻ�����֣���ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ���
R��CH2CH2OH���ۻ�����F�����ϵ�һ�ȴ���ֻ�����֣���ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ���
��ش��������⣺
(1)A�Ľṹ��ʽΪ ��B���������ŵ������� ��
(2)C������Ϊ ��E�ķ���ʽΪ ��
(3)A��B��C��D��F��G�ķ�Ӧ���ͷֱ�Ϊ �� �� ��
(4)д�����з�Ӧ�Ļ�ѧ����ʽ��
D��E�ڢٲ���Ӧ ��
F��G ��
(5)I�ĽṹͲʽΪ ��
(6)I��ͬϵ��J��I��Է�������С14��J��ͬ���칹������ͬʱ���������������ٱ�����ֻ������ȡ�������ڼ��ܷ���������Ӧ�������뱥��NaHCO3��Һ��Ӧ�ų�CO2������ �֣������������칹)��J��һ��ͬ���칹�巢��������Ӧ���ữ��˴Ź�������Ϊ����壬�ҷ������Ϊ2��2��1��д��J������ͬ���칹��Ľṹ��ʽ ��
��1��(CH3)3CCl��̼̼˫�� ��2��2������1��������C4H8O2
��3����ȥ��Ӧ��������Ӧ��ȡ����Ӧ
��4��(CH3)2CHCHO��2Cu(OH)2��NaOH (CH3)2CHCOONa��3H2O��Cu2O��
(CH3)2CHCOONa��3H2O��Cu2O�� ��5��
��5��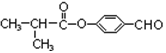
��6��18��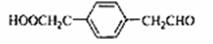 ��
��
�������������A�ķ���ʽΪC4H9Cl���˴Ź������ױ�����ֻ��һ�ֻ�ѧ�������⣬����A�Ľṹ��ʽΪ(CH3)3CCl��A��B����ȥ��Ӧ��B��(CH3)2C��CH2��B��C�Ǽӳɷ�Ӧ�������ṩ�ķ�Ӧ��Ϣ��C��(CH3)2CHCH2OH��C��D��������Ӧ��D��(CH3)2CHCHO��D��E��������Ӧ��E��(CH3)2CHCOOH��F�ķ���ʽΪC7H8O�������ϵ�һ�ȴ���ֻ�����֣�����F�Ľṹ��ʽΪ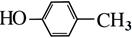 ���ڹ��������������������ʵ���֮��1:2��Ӧ���ǶԼ��еļ��е�2��H��Clȡ�������G�Ľṹ��ʽΪ
���ڹ��������������������ʵ���֮��1:2��Ӧ���ǶԼ��еļ��е�2��H��Clȡ�������G�Ľṹ��ʽΪ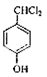 ��G��H��ˮ�ⷴӦ��������Ϣ��Ӧ���ǡ�CHCl2��ɡ�CHO����H�Ľṹ��ʽΪ
��G��H��ˮ�ⷴӦ��������Ϣ��Ӧ���ǡ�CHCl2��ɡ�CHO����H�Ľṹ��ʽΪ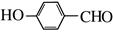 ��H��I��������Ӧ��I�Ľṹ��ʽΪ��
��H��I��������Ӧ��I�Ľṹ��ʽΪ��  ��
��
��1���������Ϸ�����֪��A�Ľṹ��ʽΪ(CH3)3CCl��B���������ŵ�������̼̼˫����
��2��C������Ϊ2������1��������E�ķ���ʽΪC4H8O2��
��3��A��B��C��D��F��G�ķ�Ӧ���ͷֱ�Ϊ��ȥ��Ӧ��������Ӧ��ȡ����Ӧ��
��4��ȩ��������������ͭ����Һ��Ӧ�Ļ�ѧ����ʽΪ(CH3)2CHCHO��2Cu(OH)2��NaOH (CH3)2CHCOONa��3H2O��Cu2O���������ڹ�������������������������ԭ�ӵ�ȡ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ
(CH3)2CHCOONa��3H2O��Cu2O���������ڹ�������������������������ԭ�ӵ�ȡ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ ��
��
��5��I�Ľṹ��ʽΪ��  ��
��
��6��J��I��ͬϵ���Է�������С14��˵��J��I��һ��Cԭ�ӣ�����ȡ���������ܷ���������Ӧ�����ܺͱ���NaHCO3��Һ��Ӧ�ų�CO2��������һ�����Ȼ���һ��ȩ������COOH�롪CH2CH2CHO��ϣ���COOH�롪CH(CH3)CHO��ϣ���CH2COOH�롪CH2CHO��ϣ���CH2CH2COOH�롪CHO��ϣ���CH(CH3)COOH�롪CHO��ϣ���HOOCCH��CHO�����롪CH3����ϣ�ÿһ����Ͽ����ڡ��䡢������λ�ñ仯��һ����6��3=18������������ͬ���칹�塣���У�һ��ͬ���칹�巢��������Ӧ���ữ��˴Ź�������Ϊ����壬�ҷ������Ϊ2:2:1���ṹ��ʽΪ�� ��
��
���㣺�����л�������֪ʶ���漰�л��������л��ṹ��ʽ������ʽ����ѧ����ʽ��ͬ���칹�������ж�

 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
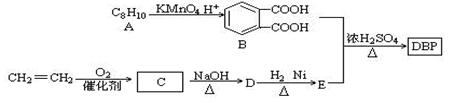
����С����ҵ��ϵ�д���������һ������ƫͷʹ����Ч��ҩ��������ij�о�С�鿪�������������صĺϳ�·�ߡ�
��֪:��A����Է�������Ϊ104,1 mol A��������̼�����Ʒ�Ӧ����44.8 L���壨��״����;
��B�Ľṹ�к���ȩ��;
��C��һ�������������л���D;
��RCHO+HOOC��CH2COOH RCH
RCH C��COOH��2+H2O��RCH
C��COOH��2+H2O��RCH C��COOH��2
C��COOH��2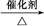 RCH
RCH CHCOOH+CO2����
CHCOOH+CO2����
��ش��������⡣
��1��A�ķ���ʽ����������,B�Ľṹ��ʽΪ����������
��2��C���ܷ����ķ�Ӧ����������������ţ���
| A��������Ӧ | B��ˮ�ⷴӦ |
| C����ȥ��Ӧ | D��������Ӧ |
��4��E����������������
��5����������������D��ͬ���칹�干������������,�����ں˴Ź���������ֻ�������������ʵĽṹ��ʽΪ����������
�ٱ�����ֻ������ȡ����;
�ڱ����ϵ�һ�ȴ���ֻ������;
��1 mol��ͬ���칹����������̼�����Ʒ�Ӧ����2 mol CO2��

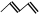
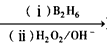 R-CH2CH2OH
R-CH2CH2OH 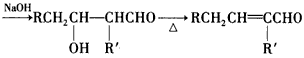


 L��Ũ��ˮ�����µ����������Ϊ_________
L��Ũ��ˮ�����µ����������Ϊ_________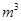 ��
��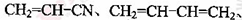 ��
�� ����д��ABS�Ľṹ��ʽ_______________________________��
����д��ABS�Ľṹ��ʽ_______________________________��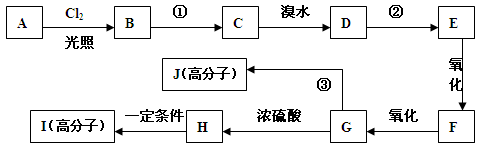
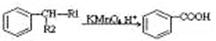
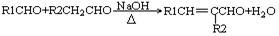 ����R1����R2��ʾ��ԭ�ӻ�������
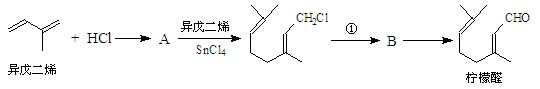
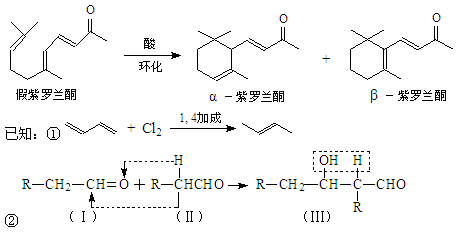
����R1����R2��ʾ��ԭ�ӻ������� )�����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɡ�һ�ֺϳ�·�����£�
)�����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɡ�һ�ֺϳ�·�����£�