��Ŀ����
���û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��1���ϳɰ���ӦN2(g)��3H2(g) 2NH3(g)���ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�������������ҡ���������ʹ�ô���ʹ��Ӧ�ġ�H �����������С�����ı䡱����
2NH3(g)���ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�������������ҡ���������ʹ�ô���ʹ��Ӧ�ġ�H �����������С�����ı䡱����
��2����25���£���Ũ�Ⱦ� Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ��������ֲ�������
Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ��������ֲ�������
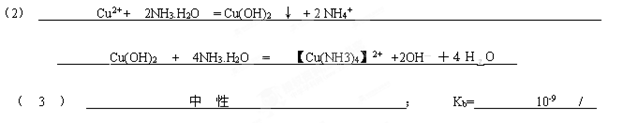
�������ɳ��������ӷ���ʽΪ __________________
��ˮ���������ɵij����Ჿ���ܽ⣬д���ܽ���������ӷ���ʽ__________________________.
( ��֪25��ʱKsp[Mg(OH)2]=1.8��10-11�� KsP[Cu(OH)2]=2.2��10- 20 )
20 )
��3����25���£���a mol��L-1�İ�ˮ��0.01 mol��L-1������������ϣ���Ӧ����Һ��
c(NH4��) = c(Cl��)������Һ�� _____ �ԣ���ᡱ��������С��������ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb= ��
��4) ��һ���̶��ݻ�Ϊ5L���ܱ������г���0.20 mol SO2��0.10molO2������Ӻ�ﵽƽ�⣬��������к�SO3 0.18mol����v(o2)= mol.L-1.min-1��������ͨ��0.20mol SO2��0.10mol O2���ٴδﵽƽ��� n(SO3)��ȡֵ��ΧΪ________________��
��1���ϳɰ���ӦN2(g)��3H2(g)
 2NH3(g)���ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�������������ҡ���������ʹ�ô���ʹ��Ӧ�ġ�H �����������С�����ı䡱����
2NH3(g)���ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�������������ҡ���������ʹ�ô���ʹ��Ӧ�ġ�H �����������С�����ı䡱������2����25���£���Ũ�Ⱦ�
 Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ��������ֲ�������
Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ��������ֲ��������������ɳ��������ӷ���ʽΪ __________________
��ˮ���������ɵij����Ჿ���ܽ⣬д���ܽ���������ӷ���ʽ__________________________.
( ��֪25��ʱKsp[Mg(OH)2]=1.8��10-11�� KsP[Cu(OH)2]=2.2��10-
 20 )
20 )��3����25���£���a mol��L-1�İ�ˮ��0.01 mol��L-1������������ϣ���Ӧ����Һ��
c(NH4��) = c(Cl��)������Һ�� _____ �ԣ���ᡱ��������С��������ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb= ��
��4) ��һ���̶��ݻ�Ϊ5L���ܱ������г���0.20 mol SO2��0.10molO2������Ӻ�ﵽƽ�⣬��������к�SO3 0.18mol����v(o2)= mol.L-1.min-1��������ͨ��0.20mol SO2��0.10mol O2���ٴδﵽƽ��� n(SO3)��ȡֵ��ΧΪ________________��
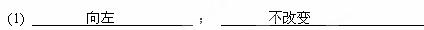
(a-0.01)_______________________ ��
��4) _0.036___ ___ �� _______0.40>n(SO3)> 0.36__________________��
��

��ϰ��ϵ�д�
�����Ŀ
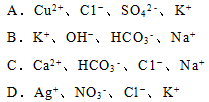
 ��CH3COO����
��CH3COO����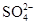 ��
��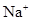
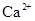 ��
��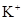 ��
��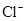 ��
��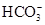
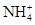 ��
��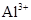 ��
��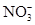 ��
��