��Ŀ����
18��A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ��������������A�ǵؿ��к�����ߵ�Ԫ�أ�C�ǵؿ��к�����ߵĽ���Ԫ�أ�A��Eͬ���壮DԪ��ԭ�������������Ǵ�����������һ�룮B��C��Ԫ��ԭ�ӵ�����������֮�͵���DԪ��ԭ�ӵ�������������C��D��Ԫ��ԭ������������֮�͵���FԪ��ԭ�ӵ��������������ش��������⣺
��1��Ԫ��A��Ԫ�����ڱ��е�λ���ǵڶ����ڵڢ�A�壮
��2��д��B��E��Ԫ���γɻ�����ĵ���ʽ��
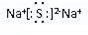 ��������ѧ���������Ӽ���
��������ѧ���������Ӽ�����3��A��D��E��F����̬�⻯����ȶ���˳��H2O��HCl��H2S��SiH4���û�ѧʽ��ʾ��
��4��д��C��E��Ӧ������������ˮ�������Ӧ�Ļ�ѧ����ʽΪ��2Al��OH��3+3H2SO4�TAl2��SO4��3+6H2O
��5��C��D����������ֱ���B���������ﷴӦ�����ӷ���ʽ��Al2O3+2OH-�T2AlO2-+H2O��SiO2+2OH-�TSiO32-+H2O��
���� A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ��������������A�ǵؿ��к�����ߵ�Ԫ�أ���AΪ��Ԫ�أ�C�ǵؿ��к�����ߵĽ���Ԫ�أ���CΪ��Ԫ�أ�A��Eͬ���壬��EΪ��Ԫ�أ�DԪ��ԭ�������������Ǵ�����������һ�룬��DΪ��Ԫ�أ�B��C��Ԫ��ԭ�ӵ�����������֮�͵���DԪ��ԭ�ӵ���������������B�������1�����ӣ�ԭ����������������֮�䣬����BΪ��Ԫ�أ�C��D��Ԫ��ԭ������������֮�͵���FԪ��ԭ�ӵ���������������FΪ��Ԫ�أ��ݴ˴��⣮
��� �⣺A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ��������������A�ǵؿ��к�����ߵ�Ԫ�أ���AΪ��Ԫ�أ�C�ǵؿ��к�����ߵĽ���Ԫ�أ���CΪ��Ԫ�أ�A��Eͬ���壬��EΪ��Ԫ�أ�DԪ��ԭ�������������Ǵ�����������һ�룬��DΪ��Ԫ�أ�B��C��Ԫ��ԭ�ӵ�����������֮�͵���DԪ��ԭ�ӵ���������������B�������1�����ӣ�ԭ����������������֮�䣬����BΪ��Ԫ�أ�C��D��Ԫ��ԭ������������֮�͵���FԪ��ԭ�ӵ���������������FΪ��Ԫ�أ�
��1��AΪ��Ԫ�أ���Ԫ�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬
�ʴ�Ϊ���ڶ����ڵڢ�A�壮
��2��B��E��Ԫ���γɻ�����Ϊ���ƣ����ĵ���ʽΪ��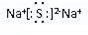 ��������ѧ������Ϊ���Ӽ���
��������ѧ������Ϊ���Ӽ���
�ʴ�Ϊ��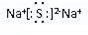 �����Ӽ���
�����Ӽ���
��3��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ������ڷǽ�����O��Cl��S��Si��������̬�⻯����ȶ���˳��ΪH2O��HCl��H2S��SiH4��
�ʴ�Ϊ��H2O��HCl��H2S��SiH4��
��4��д��C��E��Ӧ������������ˮ����ֱ�Ϊ�������������ᣬ�������Ӧ�Ļ�ѧ����ʽΪ2Al��OH��3+3 H2SO4�TAl2��SO4��3+6H2O��
�ʴ�Ϊ��2Al��OH��3+3 H2SO4�TAl2��SO4��3+6H2O��
��5��C��D��������ֱ�Ϊ�������Ͷ������裬���Ƿֱ����������Ʒ�Ӧ�����ӷ���ʽΪAl2O3+2OH-�T2AlO2-+H2O��SiO2+2OH-�TSiO32-+H2O��
�ʴ�Ϊ��Al2O3+2OH-�T2AlO2-+H2O��SiO2+2OH-�TSiO32-+H2O��
���� ������Ҫ������Ԫ�����ڱ�������ʽ��Ԫ�������ɡ�����Ԫ�ػ�����֪ʶ���ѶȲ���Ԫ���ƶ��ǽ���Ĺؼ�������ʱע�����֪ʶ��������ã�

 һ����������ϵ�д�
һ����������ϵ�д�| A�� | �� | B�� | �ڢۢ� | C�� | �ڢ� | D�� | �٢ڢۢ� |
| A�� | 146C��������Ϊ14 | B�� | 146C��������Ϊ14 | ||
| C�� | 146C��126C��Ϊͬλ�� | D�� | 146C��Ħ������Ϊ14 |
�����Ĵ�С ��������Ŀ ����֮��ľ��룮
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �٢ڢ� |

| A�� | CO��H2�ϳ�CH3OH�ķ�Ӧ����H��0����S��0 | |
| B�� | �����¶Ȳ��䣬�ٳ���1molCO��2molH2����Ӧ�ﵽ��ƽ��ʱ$\frac{n��CHOH��}{n��CO��}$��С | |
| C�� | ͼ1������b�ɱ�ʾʹ���˴����������仯��� | |
| D�� | ͼ3�����߿ɱ�ʾ�ڲ�ͬѹǿP1��P2��P1��P2�������¼״��ٷֺ������¶ȱ仯����� |
| A�� | �����ʵ������������������ֱ���ȫȼ�գ����߷ų������� | |
| B�� | ��H+��aq��+OH-��aq��=H2O��I������H=-57.3kJ•mol-1molCH3COOH��ϡ��Һ�뺬l mol NaOH��ϡ��Һ��ϣ��ų�������С��57.3 kJ | |
| C�� | ��C��ʯī��=�����ʯ������H=+1.90kJ•mol-1��֪�����ʯ��ʯī�ȶ� | |
| D�� | 500�桢30MPa�£���0.5 mol��g����1.5 mol ��g�������ܱ������г�ַ�Ӧ���ɣ�g��������19.3 kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g������H=-38.6kJ•mol-1 |
| A�� | �����£�7.8g����Na2O2�У����е�������������Ϊ0.4 NA | |
| B�� | 4.48L���������к�0.6NA��N-H�� | |
| C�� | ����CuƬ��ϡ������ɵ�ԭ��أ�����������������5.6gʱ���������·�ĵ���Ϊ0.3NA | |
| D�� | 4��ʱ��20g2H216O�к��й��õ��Ӷ���Ϊ2NA |
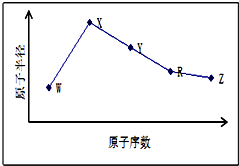 W��X��Y��Z��R�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y������������Ӧˮ������ҽ��θ���кͼ��е�һ�֣�R��W������ͬ��������������Z��ͬ��������Ԫ����ԭ�Ӱ뾶��С��
W��X��Y��Z��R�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y������������Ӧˮ������ҽ��θ���кͼ��е�һ�֣�R��W������ͬ��������������Z��ͬ��������Ԫ����ԭ�Ӱ뾶��С��