��Ŀ����
����Ŀ��������Ϣ�ش��������⣺
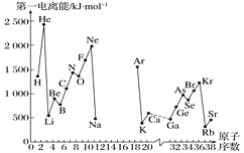
A����һ������I1��ָ��̬ԭ��X(g)���ڻ�̬ʱ��ʧȥһ�����ӳ�Ϊ��̬������X��(g)����������������ͼ�Dz���Ԫ��ԭ�ӵĵ�һ������I1��ԭ�������仯������ͼ(����12����17��Ԫ�ص��й�����ȱʧ)��
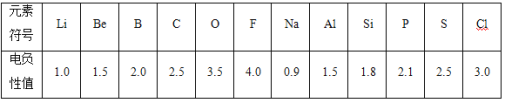
B����ͬԪ�ص�ԭ���ڷ������������ӵ�������С������ֵ��ʾ������ֵ��Ϊ�縺�ԡ�һ����Ϊ����������ɼ�ԭ�Ӽ�ĵ縺�Բ�ֵ����1.7��ԭ��֮��ͨ���γ����Ӽ�����������ɼ�ԭ�Ӽ�ĵ縺�Բ�ֵС��1.7��ͨ���γɹ��ۼ����±���ijЩԪ�صĵ縺��ֵ��
��1�����������ϢAͼ��ͬ����Ԫ�ص�һ�����ܵı仯���ɣ��ƶϵ�������Na��Ar�⼸��Ԫ���У�Al�ĵ�һ�����ܵĴ�С��ΧΪ______��Al��________(��Ԫ�ط���)��
��2������ϢAͼ�з�����֪��ͬһ����Ԫ��ԭ�ӵĵ�һ������I1�ı仯������______________��
��3����ϢAͼ�е�һ��������С��Ԫ�������ڱ��е�λ����_______����__________�塣
��4�����ݶԽ��߹���Be��AlԪ������������Ӧˮ������������ƣ����Ƕ�����_______�ԣ�����Be(OH)2��ʾ�������ʵ����ӷ���ʽ��____________��
��5��ͨ�������縺��ֵ�ı仯���ɣ�ȷ��MgԪ�صĵ縺��ֵ����С��Χ_________��
��6�������Ԫ�صĵ縺�Ժͽ����ԡ��ǽ����ԵĹ�ϵ��__________��
��7���ӵ縺�ԽǶȣ��ж�AlCl3�����ӻ����ﻹ�ǹ��ۻ�����___________��˵�����ɲ�д���жϵķ���_________��
���𰸡�Na Mg ���ϵ������μ�С ���� �ڢ�A �� Be(OH)2��2H��=Be2����2H2O��Be(OH)2��2OH��=BeO22-��2H2O 0.9��1.5 �ǽ�����Խǿ���縺��Խ������Խǿ���縺��ԽС AlԪ�غ�ClԪ�صĵ縺�Բ�ֵΪ1.5��С��1.7�������γɹ��ۼ���Ϊ���ۻ����� ���Ȼ������ȵ�����̬�����е�����ʵ�飬��������磬˵���ǹ��ۻ�����
��������
������Ҫ����Ԫ�������ɺ����ʵĻ�ѧ���ʡ�
(1) ����Ϣ������ͼ���Կ�����ͬ���ڵ��� A ��Ԫ�صĵ�һ��������С�������� A ��Ԫ�صĵ�һ������С���� A ��Ԫ�صĵ�һ�����ܣ��� Na��Al��Mg ��
(2) ��ͼ�пɿ���ͬ����Ԫ�صĵ�һ�����ܴ��ϵ�����С��
(3) ���ݵ�һ�����ܵĵݱ���ɿ��Կ�����ͼ������Ԫ���� Rb �ĵ�һ��������С���������ڱ��е�λ��Ϊ�������ڵ���A �壻
(4) ���ݶԽ��߹��� ![]() ��
��![]() ���������ƣ�
���������ƣ� ![]() Ӧ�������ԣ����ݿ������Ƶ�д��
Ӧ�������ԣ����ݿ������Ƶ�д��![]() ���ᡢ�Ӧ�����ӷ���ʽΪBe(OH)2��2H��=Be2����2H2O��Be(OH)2��2OH��=BeO22-��2H2O��
���ᡢ�Ӧ�����ӷ���ʽΪBe(OH)2��2H��=Be2����2H2O��Be(OH)2��2OH��=BeO22-��2H2O��
(5) ���ݵ縺�Եĵݱ���ɣ�ͬ����Ԫ�أ������ҵ縺��������ͬ����Ԫ�ش��ϵ��µ縺����С��֪����ͬ�����е縺��Na��Mg ��Al��Be��Mg��Ca����С��ΧӦΪ0.9��1.5��
(6) ��縺�Կ�����������ԭ���������������Ĵ�С�����Ե縺��Խ��ԭ���������ӵ�����Խǿ���ǽ�����Խǿ����֮������Խǿ��
(7)![]() �� Al �� Cl �ĵ縺�Բ�ֵΪ 1.5 ��������Ϣ���縺�Բ�ֵ��С�� 1.7 �����γɹ��ۼ�������
�� Al �� Cl �ĵ縺�Բ�ֵΪ 1.5 ��������Ϣ���縺�Բ�ֵ��С�� 1.7 �����γɹ��ۼ�������![]() Ϊ���ۻ�������ӻ�����������״̬����������ʽ���ڣ����Ե��磬�����ۻ����ﲻ�ܵ��硣
Ϊ���ۻ�������ӻ�����������״̬����������ʽ���ڣ����Ե��磬�����ۻ����ﲻ�ܵ��硣

 ��У����ϵ�д�
��У����ϵ�д�


