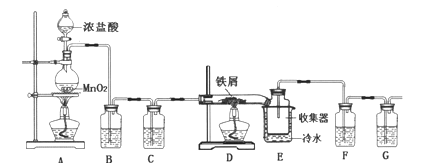
��Ŀ����
����Ŀ�����������Ƿdz���Ҫ���л��ܼ��ͻ���ԭ�ϣ���ͼת����ϵ�еĢ١��ڡ����ǹ�ҵ�Ϻϳ����������ij�����Ӧ������A��һ�ֳ����ĵ�ζƷ����ش�
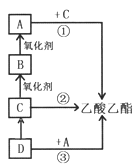
��1���л���A�к��еĹ�����������____________��
��2��д����Ӧ�ٵĻ�ѧ����ʽ_____________��
��3����֪��Ӧ���ڴ����������������������Ӧ�T��һ���ϳɲ��ͬʱ����һ����ɫ��ζ���壬������Ļ�ѧʽΪ_________��
��4������˵����ȷ����_________
A.��Dͨ����ˮ�У���ˮ��Ϊ��ɫ��ԭ����D��Br2������ȡ����Ӧ
B.C![]() B��B
B��B![]() A��������������ͬ��Ҳ���Բ�ͬ
A��������������ͬ��Ҳ���Բ�ͬ
C.��Ӧ���Ǣ١��ڡ�����Ψһ������ɫ��ѧ�ġ����ŷš�Ҫ���
D.��֪������B����ͨ��һ����Ӧ����������������ͬʱ��ˮ����
���𰸡� �Ȼ� CH3COOH+C2H5OH![]() CH3COOC2H5+H2O��2�֣�����δдȫ��1�֣� H2 B C
CH3COOC2H5+H2O��2�֣�����δдȫ��1�֣� H2 B C
���������������������������������Ҵ�ͨ��������Ӧ���ɣ�A��һ�ֳ����ĵ�ζƷ��A�����ᣬ��C���Ҵ���B����ȩ��D����ϩ����Ӧ������������ȡ������������Ӧ�����Ҵ����ⷨ��ȡ������������Ӧ������ϩ�ӳɷ���ȡ����������
��������1���л���A�����к��еĹ������������Ȼ�����2����Ӧ�����ᡢ�Ҵ��������������Ļ�ѧ����ʽΪCH3COOH+C2H5OH![]() CH3COOC2H5+H2O����3����Ӧ�����Ҵ����ⷨ��ȡ����������������ɫ��ζ����Ļ�ѧʽΪH2����4��A.����ϩͨ����ˮ�У���ˮ��Ϊ��ɫ��ԭ������ϩ��Br2�����˼ӳɷ�Ӧ����A����
CH3COOC2H5+H2O����3����Ӧ�����Ҵ����ⷨ��ȡ����������������ɫ��ζ����Ļ�ѧʽΪH2����4��A.����ϩͨ����ˮ�У���ˮ��Ϊ��ɫ��ԭ������ϩ��Br2�����˼ӳɷ�Ӧ����A����
B.�Ҵ�![]() ��ȩ����ȩ
��ȩ����ȩ![]() �����������������������Ҳ���Բ��ǣ���B��ȷ��
�����������������������Ҳ���Բ��ǣ���B��ȷ��
C.��Ӧ����ԭ��������100%����C��ȷ��
D.����B�ķ���ʽC2H4O�����������ķ���ʽC4H8O2��������B����ͨ��һ����Ӧ��������������û�������������ɣ���D����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
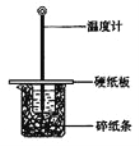
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ��������� ��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ��ƫС����Ӱ�족)
��3�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��ȡ�H_____ (���ȡ�����ȡ�)
��4���������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬������к��ȡ�H�� (�ƫ����ƫС��������Ӱ�족) ����KOH����NaOH��ʵ�������������_________________(�ƫ����ƫС��������Ӱ�족)��
��5�����Ǽ�¼��ʵ���������£�
ʵ����Ʒ[ | ��Һ�¶� | �к��� ��H | |||
��ʼƽ ���¶� | ��Ӧ����¶� | ||||
�� | 50mL��0.55mol.L-1NaOH | 50mL��0.5mol.L-1HCl | 20�� | 23.3�� | |
�� | 50mL��0.55mol.L-1NaOH | 50mL��0.5mol.L-1HCl | 20�� | 23.5�� | |
��֪��Ӧ����Һ�ı�����CΪ4.18KJ����-1�� Kg-1�������ʵ��ܶȾ���Ϊ1g��cm-3��
�ٸ����ϱ����ݼ����к��ȡ�H=__________(������С�����һλ)
�ڸ���ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ�� ��
��6������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)_______________��
A��ʵ��װ�ñ��¡�����Ч����
B����ȡϡ������Һ�����ʱ���Ӷ���
C��һ����NaOH��Һ����ʢ�������С�ձ���
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨHCl��Һ���¶�