��Ŀ����
����Ŀ����Ҫ��ش���������:
��1�������м������ʣ��뽫����������пո��ڣ�
A��CH2=CH-COOH�����ᣨC17H33COOH��
B��C60��ʯī
C��![]() ��
��![]()
D��35Cl��37Cl
E���Ҵ����Ҷ���
�ٻ�Ϊͬλ�ص���______________��
�ڻ�Ϊͬϵ�����_________________��
�ۻ�Ϊͬ�����������__________��
�ܻ�Ϊͬ���칹�����_____________��
��2���ݶ��ݣ�TNT���ṹ��ʽΪ____________________________��
��3��ij�л��ۺ�����ṹΪ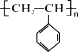 ���Իش��������⣺
���Իش��������⣺
���л���������___________________������Ϊ______��
��ʵ���øø߾������Է���������ƽ��ֵ��Ϊ52000����ø߾���ľۺ϶�nΪ________��
��4��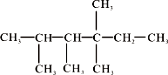
�����������__________________________��
�ڴ��л���Ϊϩ���ӳɵIJ����ԭ��ϩ���Ľṹ������_______�֡�
��5��ij���ʽṹ��ͼ��ʾ������ʽΪ_________________�������ʿ���������_____���������������Ӧ��
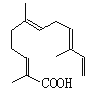
A������KMnO4��Һ B������
C����ˮ D��NaOH��Һ
���𰸡�D A B C 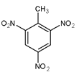 �۱���ϩ
�۱���ϩ ![]() 500 2,3, 4,4���ļ����� 4 C15H22O2 ABCD
500 2,3, 4,4���ļ����� 4 C15H22O2 ABCD
��������
(1). ��������ͬ����������ͬ��ԭ�ӻ�ͬһԪ�صIJ�ͬ���ػ�Ϊͬλ�أ��ṹ���ơ��������������ɸ���CH2��ԭ���ŵĻ����ﻥ��Ϊͬϵ����Ԫ����ͬ���ṹ�����ʲ�ͬ�ĵ��ʻ�Ϊͬ�������壻������ͬ����ʽ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壻
(2). TNT���������ױ����׳ƣ�
(3). �ɽṹ��ʽ��֪���л���Ϊ�۱���ϩ���߷��ӻ��������ظ����ֵĽṹ��Ԫ�����ڣ����ݾ۱���ϩ����Է�������Ϊ104n��ȷ��n��ֵ��
(4). ���л���Ϊ��������������������ԭ����н�����üӳɷ�Ӧ�ص㻹ԭC=C������̼ԭ���϶�����Hԭ�ӵ�̼ԭ�Ӽ���Ի�ԭC=C��
(5). �������ʵĽṹ��ʽ�ж������ʽ�����л����к���̼̼˫�����Ȼ����ֹ��������ݴ��ж�����ܷ����ķ�Ӧ��
(1). ��. 35C1��37Cl��������ͬ����������ͬ����Ϊͬλ�أ��ʴ�Ϊ��D��
��. CH2=CH-COOH�����ᣨC17H33COOH���ṹ���ơ�����������15����CH2��ԭ���ţ���Ϊͬϵ��ʴ�Ϊ��A��
��. C60��ʯī������̼Ԫ����ɵIJ�ͬ���ʣ���Ϊͬ�������壬�ʴ�Ϊ��B��
��. ![]() ��
��![]() ����ʽ��ͬ�����ṹ��ͬ����Ϊͬ���칹�壬�ʴ�Ϊ��C��
����ʽ��ͬ�����ṹ��ͬ����Ϊͬ���칹�壬�ʴ�Ϊ��C��
(2). TNT���������ױ����׳ƣ����ɼױ�����������Ӧ�Ƶã���ṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(3). ��.�ɽṹ��ʽ![]() ��֪���л���Ϊ�۱���ϩ���߷��ӻ��������ظ����ֵĽṹ��Ԫ����������۱���ϩ������Ϊ
��֪���л���Ϊ�۱���ϩ���߷��ӻ��������ظ����ֵĽṹ��Ԫ����������۱���ϩ������Ϊ![]() ���ʴ�Ϊ���۱���ϩ��
���ʴ�Ϊ���۱���ϩ��![]() ��
��
��. �۱���ϩ����Է�������Ϊ104n������104n=52000��n=500���ʴ�Ϊ��500��
(4). ��. ���л���Ϊ��������������̼������6��Cԭ�ӣ�����Ϊ���飬���ʱ��������ȡ�����ı��֮����С�����Ӿ���ȡ���������һ�˿�ʼ��ţ����л���Ӧ�ô���˿�ʼ��ţ���2��3��Cԭ���ϸ�����1��������4��Cԭ���Ϻ���2���������л�������Ϊ��2,3,4,4-�ļ����飻�ʴ�Ϊ��2,3,4,4-�ļ����飻
��. ���ݼӳɷ�Ӧ���ص㻹ԭC=C������̼ԭ���϶�����Hԭ�ӵ�̼ԭ�Ӽ���Ի�ԭΪC=C����ͼ ��ʾ��5��λ�ÿ��Ի�ԭΪC=C������λ��1��5��ͬ����ԭ��ϩ���Ľṹ������4�֣��ʴ�Ϊ��4��
��ʾ��5��λ�ÿ��Ի�ԭΪC=C������λ��1��5��ͬ����ԭ��ϩ���Ľṹ������4�֣��ʴ�Ϊ��4��
(5). �ɸ��л���Ľṹ��ʽ��֪����һ�������к���15��̼ԭ�ӡ�22��Hԭ�Ӻ�2��Oԭ�ӣ������ʽΪC15H22O2�����л��������Ĺ�����Ϊ̼̼˫�����Ȼ�������̼̼˫�������ʿ��Ա����Ը��������Һ���������Ժ���������ˮ�����ӳɷ�Ӧ�������Ȼ������ʿ��Ժ�����������Һ�����кͷ�Ӧ���ʴ�Ϊ��C15H22O2��ABCD��

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�����Ŀ������ʵ�����������ͽ��۾���ȷ����
ѡ�� | ʵ����� | ���� | ���� |
A | ��ͭ�ۼ���1.0mol/LFe2(SO4)3��Һ�� | ��Һ��Ϊ��ɫ | ��������ͭ���� |
B | ������ǯ��סһС����ɰֽ��ϸ��ĥ���������ھƾ����ϼ��� | �ۻ����Һ̬������������ | ���������۵���������۵� |
C | �����£���pH�Ʋ�0.1mol/LNaX��Һ��0.1mol/LNa2CO3��Һ��pH | ǰ��С�ں��� | ���ԣ�HX>H2CO3 |
D | ��10%��������Һ�м�������ϡ���ᣬˮԡ����һ��ʱ�䣬�ټ���������Һ | δ���ֹ������� | ����δ����ˮ�� |
A. A B. B C. C D. D






