��Ŀ����
����Ŀ����������������Դ�����ˮ�����Ⱦ�ǻ�����������Ҫ�о����⡣
(1) ��ѧ�ϲ���NH3����NxOy��������������Ⱦ��������Ϊ��ҵ������������Դ��
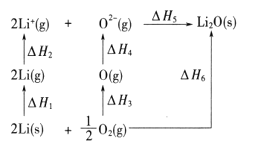
��֪��2NO(g)=N2(g)+O2(g) ��H=��177kJ/mol
4NH3(g)+3O2(g)===2N2(g)+6H2O(g) ��H=��1253.4kJ/mol
����NH3����NO���ɵ�������̬ˮ���Ȼ�ѧ����ʽΪ___________________��
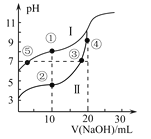
(2)��֪��N2(g)+3H2(g) ![]() 2NH3(g) ��H<0����ͬ�¶��£������������зֱ�Ͷ����ͬ���ķ�Ӧ����з�Ӧ����ò�ͬѹǿ��ƽ��������NH3�����ʵ���������ͼ��ʾ��
2NH3(g) ��H<0����ͬ�¶��£������������зֱ�Ͷ����ͬ���ķ�Ӧ����з�Ӧ����ò�ͬѹǿ��ƽ��������NH3�����ʵ���������ͼ��ʾ��
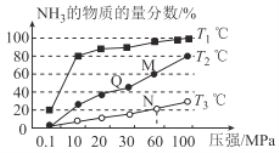
��M���v��_________Q���v��(����>����<������=��)��
��T3�¶��£���1molN2��3molH2����2L���ܱ������У�ά��ѹǿΪ60MPa���䣬�ﵽN���ƽ��״̬����Ӧ��Ũ��ƽ�ⳣ��K=_____________ (����������ʾ)��M���ƽ�ⳣ����N���ƽ�ⳣ��_________(����������С�����������)��
(3)ˮ���й�������(��NH3��ʾ)�ᵼ��ˮ�帻Ӫ������
���ô������Ƴ�ȥ������ԭ����ͼ��ʾ��д���ܷ�Ӧ��ѧ����ʽ��_____________��


��ȡһ�����ĺ�������ˮ���ı����������Ƶ���������Ӧһ��ʱ�����Һ�а���ȥ���ʡ��ܵ�(��Һ�����п����Եĺ����������е�Ԫ�ص�����)ȥ�����Լ�ʣ��������Ƶĺ�����m(NaClO)��m(NH3)�ı仯�������ͼ��ʾ����Bʣ��NaClO�������ڵ�A��ԭ����____����m(NaClO)��m(NH3)>7.6ʱ��ˮ�����ܵ�ȥ���ʷ����½������ܵ�ԭ����__________��
(4)�缫����Ĥ��������ǵ绯ѧ�����﹤�յ���ϡ�ij����Ĥ�����õ������Ļ���ԭ�ӽ�NO3-��ԭΪN2������ԭ������ͼ��ʾ�����������ɱ�״����2.24 L���壬�����Ͽɳ�ȥNO3-�����ʵ���Ϊ_____mol��

���𰸡�4NH3(g)��6NO (g)=5N2(g)��6H2O(g)�� ��H��-1784.4kJ��mol��1 > 25/108 �� 2NH3��3NaClO=N2��3NaCl��3H2O ����NaClO��������Ӧ���ʼӿ죬��ͬʱ����NaClO���Ķ� �в���NH3��������NO2-��NO3- 0.08
��������
(1)NH3��NO���ɵ�������̬ˮ�Ļ�ѧ����ʽΪ4NH3(g)+6NO(g)�T5N2(g)+6H2O(g)��������֪�Ȼ�ѧ����ʽ��ϸ�˹���ɷ������
(2)��Q��M������ͬһ���������ϣ���M��ѹǿ�ߣ�����ѹǿ�Ի�ѧ��Ӧ����Ӱ���𣻢���������ʽ����N���������ʵ������ٸ������ʵ���֮�ȵ������֮�ȼ���ƽ��ʱ���������ת��Ϊ�����ʵ�Ũ�ȴ���ƽ�ⳣ������ʽ����K���÷�Ӧ�����Ƿ��ȷ�Ӧ���¶�Խ��ƽ�ⳣ��KԽС���ݴ˷������
(3)�ٸ���ͼʾ�жϷ���������ԭ��Ӧ�ķ�Ӧ������������ת�����غ��Ԫ���غ������д�������Ӵ������ƻ�ӿ췴Ӧ���ʣ�NaClOͶ�����Ὣ��������ΪNO3-�ȸ���̬�����ʣ�
(4)����������������ʧ�������ɵ��������������ɵ����������ת�Ƶĵ��ӵ����ʵ�������ϵ����غ���������Ͽɳ�ȥNO3-�����ʵ�����
(1)NH3��NO���ɵ�������̬ˮ�Ļ�ѧ����ʽΪ4NH3(g)+6NO(g)�T5N2(g)+6H2O(g)����2NO(g)=N2(g)+O2(g) ��H=��177kJ/mol����4NH3(g)+3O2(g)===2N2(g)+6H2O(g) ��H=��1253.4kJ/mol�����ݸ�˹���ɢ���3+�ڵõ�4NH3(g)+6NO(g)�T5N2(g)+6H2O(g)��H=(-177kJ/mol)��3+(-1253.4kJ/mol)-1784.4kJ/mol���ʴ�Ϊ��4NH3(g)+6NO(g)�T5N2(g)+6H2O(g)��H=-1784.4kJ/mol��
(2)��Q��M������ͬһ���������ϣ���M��ѹǿ�ߣ�����M�㷴Ӧ���ʴʴ�Ϊ������
�ڷ�Ӧ������ʽ��N2(g)+3H2(g)2NH3(g)
��ʼ��(mol) 1 3 0
�仯��(mol) x 3x 2x
ƽ����(mol) 1-x 3-3x 2x
ƽ��ʱ�����ĺ���Ϊ20%����![]() ��100%=20%��x=
��100%=20%��x=![]() mol��N2��H2��NH3�����ʵ����ֱ�Ϊ
mol��N2��H2��NH3�����ʵ����ֱ�Ϊ![]() mol��2mol��
mol��2mol��![]() mol�������ʵ���Ϊ(4-2��
mol�������ʵ���Ϊ(4-2��![]() )mol=
)mol=![]() mol����ѹʱ�������֮�ȵ������ʵ���֮�ȣ�����ƽ��ʱ�������Ϊ
mol����ѹʱ�������֮�ȵ������ʵ���֮�ȣ�����ƽ��ʱ�������Ϊ![]() L=
L=![]() L����c(N2)=c(NH3)=0.4mol/L��c(H2)=1.2mol/L��ƽ�ⳣ��K=
L����c(N2)=c(NH3)=0.4mol/L��c(H2)=1.2mol/L��ƽ�ⳣ��K=![]() =
=![]() =
=![]() ���÷�Ӧ������ȣ�����ƽ�������ƶ���ƽ�ⳣ��k��С������T3��T2ʱ��T3��ƽ�ⳣ��С��T2��ʱƽ�ⳣ������M��ƽ�ⳣ������N��ƽ�ⳣ�����ʴ�Ϊ��
���÷�Ӧ������ȣ�����ƽ�������ƶ���ƽ�ⳣ��k��С������T3��T2ʱ��T3��ƽ�ⳣ��С��T2��ʱƽ�ⳣ������M��ƽ�ⳣ������N��ƽ�ⳣ�����ʴ�Ϊ��![]() ����
����
(3)�ٸ���ͼʾ����ʼ̬����̬�жϷ�Ӧ����NH3������ΪN2����NaCl����ԭΪNaCl������1molN2ת��6mol���ӣ�����ת�Ƶĵ����غ㣬��Ҫ3molNaClO���ٽ��Ԫ���غ㣬��Ӧ�Ļ�ѧ����ʽΪ2NH3+3NaClO=N2+3H2O+3NaCl���ʴ�Ϊ��2NH3��3NaClO=N2��3NaCl��3H2O��
������NaClO��������Ӧ���ʼӿ죬��ͬʱ����NaClO���Ķ࣬ʹ��B��ʣ��NaClO��������A�㣻��m(NaClO)��m(NH3)��7.7ʱ��������NaClOͶ�����Ὣ��������ΪNO3-�ȸ���̬�����ʣ�δ�ܴ���Һ�г�ȥ��ʹ���ܵ���ȥ������m(NaClO)��m(NH3)���������������ʴ�Ϊ������NaClO��������Ӧ���ʼӿ죬��ͬʱ����NaClO���ĶࣻNaClOͶ�����Ὣ����NH3��������NO2-��NO3-��
(4)��������������Ӧ����Һ�е�����������ʧ�������ɵ���������״���£�2.24L���������ʵ���=![]() =0.1mol��ת�Ƶĵ���Ϊ0.1mol��4=0.4mol������ԭ�ӽ�NO3-��ԭΪN2�����ڹ�ϵNO3-��
=0.1mol��ת�Ƶĵ���Ϊ0.1mol��4=0.4mol������ԭ�ӽ�NO3-��ԭΪN2�����ڹ�ϵNO3-��![]() N2��5e-�������Ͽɳ�ȥNO3-�����ʵ���=
N2��5e-�������Ͽɳ�ȥNO3-�����ʵ���=![]() =0.08mol���ʴ�Ϊ��0.08��
=0.08mol���ʴ�Ϊ��0.08��

 �Ķ��쳵ϵ�д�
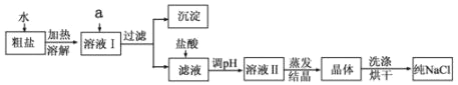
�Ķ��쳵ϵ�д�����Ŀ��ij����С��������ý���ϣ���Ҫ�ɷ�ΪNi-Al�Ͻ𣬻�������Fe��Cu��Zn���л�� �Ʊ�NiO�����ս�����Դ������������ʾ��

��֪������������1�ͱ�2��ʾ
��1�������ܵ���ʵ��ܶȻ�������25�棩
���� | Ksp | ���� | Ksp |
Fe(OH)3 | 4.0��10-38 | CuS | 6.3��10-34 |
Fe(OH)2 | 1.8��10-16 | ZnS | 1.6��10-24 |
Al(OH)3 | 1.0��10-33 | NiS | 3.2��10-18 |
Ni(OH)2 | 2.0��10-15 |
��2 ԭ�ϼ۸��
���� | �۸�/��Ԫ��-1�� |
ƯҺ����25.2%NaClO�� | 450 |
˫��ˮ����30%H2O2�� | 2400 |
�ռ��98%NaOH�� | 2100 |
�����99.5%Na2CO3�� | 600 |
��ش��������⣺
��1�������ա���Ŀ����________________________________��
��2�����Լ�a��������Ϊ__________________��ѡ����Լ���������______��
��3����������ʱ��Ӧ�����ӷ���ʽΪ__________________________________________��
��4����ʹ��Һ��Fe3+��A13+��Ũ�Ⱦ�С�ڵ���1.0��10-6 mol L-1���衰����pH������Ϊ_______________��
��5�����Լ�b��Ӧѡ��__________������ˮ��С�ʱ����Ӧ�Ļ�ѧ����ʽΪ_______________________________��
��6�����������һ��Ӧ�ù㷺�Ķ��ε�أ��ŵ�ʱ���õ�ص��ܷ�ӦΪNiOOH+MH=Ni(OH)2+M��������������2 mol����ʱ�������ϸ�����������__________g�����ʱ��������ӦʽΪ_______________________________________________��