��Ŀ����
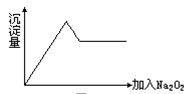
��12�֣���ʢ��5 mL 0.005 mol/L FeCl3��Һ���Թ��м���5 mL 0.01 mol/L KSCN ��Һ����Һ�ʺ�ɫ����������Һ�������ڼס�����֧�Թ��У�����Թ��м���4�α���FeCl3��Һ�������Թ��м���4��ŨKSCN��Һ��������۲���Һ��ɫ�仯��Ȼ���ٷֱ���������֧�Թ��еμ�5��0.5 mol/L NaOH��Һ���۲����ش��������⡣
������֧�Թ��зֱ���뱥��FeCl3��Һ��ŨKSCN��Һ���ɹ۲쵽��������__________________________________��
�Ʋ����������������֧�Թ��еμ�NaOH��Һ�����ɹ۲쵽��������_________
______________�������������ԭ����___________________________________��
�ǵμ�NaOH��Һ���۲쵽�������������⣬���ῴ��һ֧�Թ����г������ɣ���һ֧�Թ��м���û�С��г������ɵ��Թ���_________����ס����ҡ�����
������֧�Թ��зֱ���뱥��FeCl3��Һ��ŨKSCN��Һ���ɹ۲쵽��������__________________________________��
�Ʋ����������������֧�Թ��еμ�NaOH��Һ�����ɹ۲쵽��������_________
______________�������������ԭ����___________________________________��
�ǵμ�NaOH��Һ���۲쵽�������������⣬���ῴ��һ֧�Թ����г������ɣ���һ֧�Թ��м���û�С��г������ɵ��Թ���_________����ס����ҡ�����

��

��ϰ��ϵ�д�
�����Ŀ
 �ߡ�����
�ߡ�����
 �����¡�Fe��ˮ�����ķ�Ӧʵ�顱��ʯ���������²��ϣ�����ˮ������Ӧ����
�����¡�Fe��ˮ�����ķ�Ӧʵ�顱��ʯ���������²��ϣ�����ˮ������Ӧ����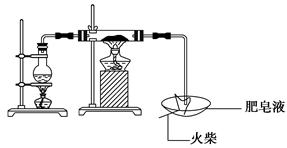
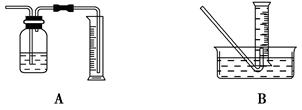
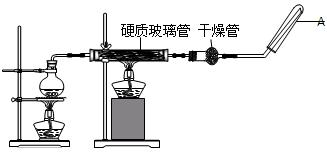
 ��
��
 2��Һ�еμ�ϡ���ᣬ��ʹ��Һ��Ϊ�ػ�ɫ
2��Һ�еμ�ϡ���ᣬ��ʹ��Һ��Ϊ�ػ�ɫ