��Ŀ����
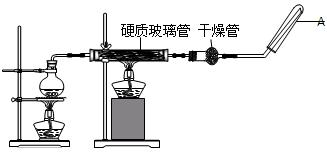
��6�֣��ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ�� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱����ش��ʵ���е����⡣
��1��д���÷�Ӧ�ķ�Ӧ����ʽ ��
��
��2��Բ����ƿ��ʢװ����ˮ����װ�����Ⱥ����Ҫ������ ����ƿ�ײ������˼�Ƭ���Ƭ���������� ���� ��
��3���Թ����ռ������� �����Ҫ��A�������ܴ���ȼ�����壬�����Ը�������� ��

��1��д���÷�Ӧ�ķ�Ӧ����ʽ
 ��
����2��Բ����ƿ��ʢװ����ˮ����װ�����Ⱥ����Ҫ������ ����ƿ�ײ������˼�Ƭ���Ƭ���������� ���� ��
��3���Թ����ռ������� �����Ҫ��A�������ܴ���ȼ�����壬�����Ը�������� ��

��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

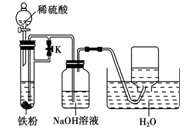

 ����������ʡ��������£�
����������ʡ��������£�
 ______________________��
______________________�� ��֮һ����ԭ����Fe3O4��4CO3Fe��4CO2������1��5 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����__________________��
��֮һ����ԭ����Fe3O4��4CO3Fe��4CO2������1��5 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����__________________��