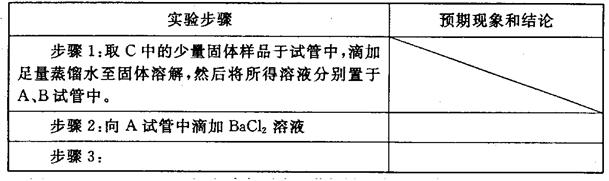
��Ŀ����
��ҵ�ϳ��ú�����SiO2��Al2O3�ĸ�����FeO Cr2O3��ұ��������Ҫ�������£�
Cr2O3��ұ��������Ҫ�������£�

��1��������л�ѧ����ʽ���ں�����д���ʵĻ�ѧʽ��ϵ������
2FeO��Cr2O3��4Na2CO3��7NaNO3 4Na2CrO4��Fe2O3��4CO2��______________��
4Na2CrO4��Fe2O3��4CO2��______________��
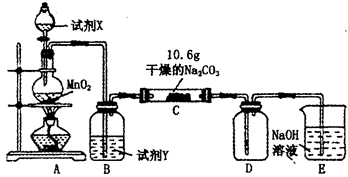
��2�������ٰ���������ϴ�ӣ���ʵ�����н���ϴ�ӳ����IJ���__________________________________�������ڿ�ѡ�õ�װ�ã����ּг�װ����ȥ����________������ţ�

��3��д���ܹ���ɲ����۵���ط�Ӧ�Ļ�ѧ����ʽ��_____________________��
��4����ѧ��������COD���ɶ���ˮ�����л�����Ⱦ�ij̶ȡ���ǿ�Ტ���ȵ������£���K2Cr2O7��ǿ����������ˮ�������ⶨ���ĵ�K2Cr2O7������Ȼ������൱��O2�ĺ�����Ϊ��ѧ����������mg/L�ƣ�����ѧ��ȤС��ⶨijˮ���Ļ�ѧ��������COD���������£�
I.ȡamLˮ��������ƿ������10��00mL 0.2500 mol/L��K2Cr2O7��Һ��
II��������
III����ָʾ������c mol �����������[ ��NH4��2Fe��SO4��
�����������[ ��NH4��2Fe��SO4�� ]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
��I����ȡK2Cr2O7��Һ��������_____________��
�ڼ����ˮ���Ļ�ѧ������ʱ���õ����й�ϵ��Ҫ��ȥ1molCr2O72�� ������___molFe2����1molCr2O72���൱��_______ molO2��
 Cr2O3��ұ��������Ҫ�������£�
Cr2O3��ұ��������Ҫ�������£�
��1��������л�ѧ����ʽ���ں�����д���ʵĻ�ѧʽ��ϵ������
2FeO��Cr2O3��4Na2CO3��7NaNO3
 4Na2CrO4��Fe2O3��4CO2��______________��
4Na2CrO4��Fe2O3��4CO2��______________����2�������ٰ���������ϴ�ӣ���ʵ�����н���ϴ�ӳ����IJ���__________________________________�������ڿ�ѡ�õ�װ�ã����ּг�װ����ȥ����________������ţ�

��3��д���ܹ���ɲ����۵���ط�Ӧ�Ļ�ѧ����ʽ��_____________________��
��4����ѧ��������COD���ɶ���ˮ�����л�����Ⱦ�ij̶ȡ���ǿ�Ტ���ȵ������£���K2Cr2O7��ǿ����������ˮ�������ⶨ���ĵ�K2Cr2O7������Ȼ������൱��O2�ĺ�����Ϊ��ѧ����������mg/L�ƣ�����ѧ��ȤС��ⶨijˮ���Ļ�ѧ��������COD���������£�
I.ȡamLˮ��������ƿ������10��00mL 0.2500 mol/L��K2Cr2O7��Һ��
II��������
III����ָʾ������c mol
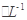 �����������[ ��NH4��2Fe��SO4��
�����������[ ��NH4��2Fe��SO4�� ]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3������I����ȡK2Cr2O7��Һ��������_____________��
�ڼ����ˮ���Ļ�ѧ������ʱ���õ����й�ϵ��Ҫ��ȥ1molCr2O72�� ������___molFe2����1molCr2O72���൱��_______ molO2��
��1��7NaNO2��2�֣�
��2����©���м�����ˮ����û��������Һ�����£��ظ�����2��3�Σ�2�֣� D��2�֣�
��3��2C2O3 4Cr��3O2����2Al��C2O3
4Cr��3O2����2Al��C2O3 2Cr��Al2O3��2�֣������𰸺���Ҳ���֣�
2Cr��Al2O3��2�֣������𰸺���Ҳ���֣�
��4������ʽ�ζ��ܻ���Һ�ܣ�1�֣� ��6 1.5��ÿ��1�֣���2�֣�
��2����©���м�����ˮ����û��������Һ�����£��ظ�����2��3�Σ�2�֣� D��2�֣�
��3��2C2O3
 4Cr��3O2����2Al��C2O3
4Cr��3O2����2Al��C2O3 2Cr��Al2O3��2�֣������𰸺���Ҳ���֣�
2Cr��Al2O3��2�֣������𰸺���Ҳ���֣���4������ʽ�ζ��ܻ���Һ�ܣ�1�֣� ��6 1.5��ÿ��1�֣���2�֣�
�������: ��1�����ݷ�Ӧǰ��Ԫ�صĻ��ϼ۱仯��֪����Ӧǰ��Ԫ�صĻ��ϼ۴ӣ�2�����ߵ���3�ۣ�ʧȥ1�����ӡ�CrԪ�صĻ��ϼ۴ӣ�3�����ߵ���6�ۣ�ʧȥ3�����ӡ���O��Na��C�Ļ��ϼ۾�û�б仯�����Ը��ݵ��ӵĵ�ʧ�غ��֪����Ԫ�صĻ��ϼ�һ�����͡�������Ԫ�ع�ʧȥ2�����ӣ�CrԪ�ع�ʧȥ12�����ӡ����7����ԭ�ӵõ�14�����ӣ���Ԫ�صĻ��ϼ۴ӣ�5�۽��͵���3�ۣ����Ի�ԭ������NaNO2��
��2�����˺�ij���ϴ�����ڹ���������ɵģ�������ʵ�����н���ϴ�ӳ����IJ�������©���м�����ˮ����û��������Һ�����£��ظ�����2��3�Σ���������Cr��OH��3�ֽ�����Cr2O3��������ȷֽ��������н��С�A��������B�Ƿ�Һ��C�ǹ��ˣ���˴�ѡD��
��3��CrԪ���Ǹ��۵����������ͨ�����ȷ�Ӧ���ⷨұ������Ӧ�ķ���ʽ�ֱ�Ϊ2C2O3
 4Cr��3O2����2Al��C2O3
4Cr��3O2����2Al��C2O3 2Cr��Al2O3��
2Cr��Al2O3����4����K2Cr2O7��Һ�������Ҿ���ǿ�����ԣ������Ҫ����ʽ�ζ��ܻ���Һ����ȡ��
��K2Cr2O7��������ԭ��Ӧ��CrԪ�صĻ��ϼ۴ӣ�6�۽��͵���3�ۣ��õ�3�����ӡ���������������Ԫ�صĻ��ϼ۴ӣ�2�����ߵ���3�ۣ�ʧȥ3�����ӡ���˸��ݵ��ӵ�ʧ�غ��֪��ȥ1molCr2O72�� ������6molFe2��������1mol�������������õ�4mol���ӣ�����molCr2O72���൱��
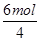 ��1.5molO2��
��1.5molO2��
��ϰ��ϵ�д�
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
�����Ŀ