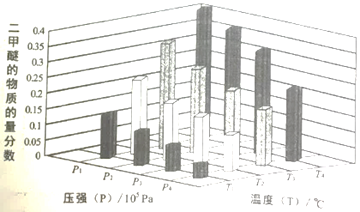
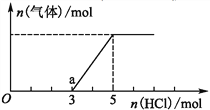
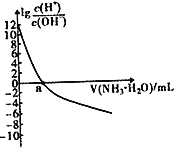
��Ŀ����
����Ŀ�����мס��ҡ�������ͬѧ�ֱ������������������Ʊ�ʵ�顣
��ͬѧ����1 mol��L��1���Ȼ�����Һ�м���������������Һ��
��ͬѧ��ֱ�Ӽ��ȱ���FeCl3��Һ��
��ͬѧ����25 mL��ˮ����μ���5��6���Ȼ���������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȡ�
�Իش��������⣺
(1)���в�����ȷ��ͬѧ��____________������ͬѧʵ���в�ֹͣ���ȣ��ῴ��___________��
(2)�������������Ʊ��Ļ�ѧ����ʽΪ_______________________________��
(3)֤�����������������������õĽ���������_______���ᴿ���������������峣�õķ�����________��
(4)�������������������ʵ�飺
�ٽ���װ��U�ι��ڣ���ʯī���缫��ͨ��һ��ʱ��������Դ���������ĵ缫����������ɫ������������������������__________(���������)��ɣ�
���������м��뱥����������Һ��������������________________________��
������������μ���ϡ���ᣬ������������__________________________��
���𰸡��� ���ֺ��ɫ���� FeCl3��3H2O![]() Fe(OH)3(����)��3HCl �����ЧӦ ������ �� �к��ɫ�������� �к��ɫ�������ɣ���������������ܽ⣬�γɻ�ɫ��Һ
Fe(OH)3(����)��3HCl �����ЧӦ ������ �� �к��ɫ�������� �к��ɫ�������ɣ���������������ܽ⣬�γɻ�ɫ��Һ
��������
��1��ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱ����ֹͣ���ȣ������������������ɽ��壬�����õ�����������ͬѧʵ���в�ֹͣ���ȣ����ڼ���ʱ�����������۳������Իῴ�����ֺ��ɫ������
��2���������Ϸ�����֪�������������Ʊ��Ļ�ѧ����ʽΪFeCl3��3H2O![]() Fe(OH)3(����)��3HCl��
Fe(OH)3(����)��3HCl��
��3��������ж����ЧӦ�����ü��������ʱ������һ�������Ĺ�·�����֤�����������������������õĽ��������Ƕ����ЧӦ�����岻������Ĥ����Һ���ԣ����ᴿ���������������峣�õķ�������������
��3����Fe(OH)3���������磬ͨ��ʱ������ɵ������������ƶ�������������ɫ������ͨ��һ��ʱ��������Դ���������ĵ缫����������ɫ���������������������������磻
���������м��뱥����������Һ���������SO42-ʹFe(OH)3���巢���۳������ɺ��ɫ��������������ʵ�����������ɺ��ɫ�ij�����
��������������������μ������ϡ������Һ���Ȼ���������������ʹFe(OH)3���巢���۳���H+ʹFe(OH)3�����ܽ⣬��˻�۲쵽�к��ɫ�������ɣ���������������ܽ⣬�γɻ�ɫ��Һ��