��Ŀ����
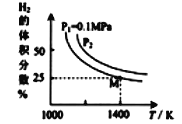
����Ŀ����A����A����A����A �ĸ�����Ԫ�ص��⻯��ķе�仯��������ͼ��ʾ������ͼ�еĵݱ���ɣ����н�������ȷ���ǣ� ��

A. CH4 ���Ӽ���������������е�ϵ�
B. ͼ�еĺ��������ֵ��ʾ��������ԭ�ӵ���������
C. ��A ��Ԫ���⻯����ȶ�������Է��������ĵ�������ǿ
D. H2O��HF��NH3 �������ʵķе��ͬ����������⻯�ﶼ�ߣ����� Ϊ������������ʵķ��Ӽ�����γ����
���𰸡�D
��������A. CH4 ���Ӽ䲻�������������е�ϵͣ���A����B. ͼ�еĺ��������ֵ��ʾ��������ԭ�ӵ�������������B����C. Ԫ�صķǽ�����Խǿ���⻯��Խ�ȶ�����A ��Ԫ���⻯����ȶ�������Է��������ĵ�����������C����D. ����DZȷ��»���ǿ��һ�ַ��Ӽ���������H2O��HF��NH3 ���Ӽ�����γ�������������ʵķе��ͬ����������⻯�ﶼ�ߣ���D��ȷ����ѡD��

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д�����Ŀ��ij��ѧ��ȤС�����������ڶ�SO2�����ʽ����о�����ش��������⣺
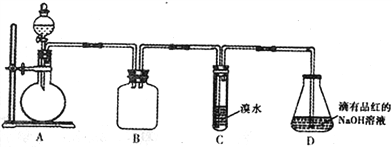
��1��װ��B��������_______________________��
��2��װ��C��Ŀ���Ǽ���SO2��_____�ԡ�װ��D��NaOHȫ��ת��ΪNaHSO3�ı�־��_______________��
��3������Ƽ�ʵ��֤������������NaHSO3��Һ��HSO3-�ĵ���ƽ�ⳣ��Ka��ˮ��ƽ�ⳣ��Kb����Դ�С��ϵ_______________________________��
��4����װ��D����NaHSO3��Һ�м���NaClO��Һʱ����Ӧ���������ֿ��ܵ������
��.HSO3-��ClO-ǡ�÷�Ӧ ��. NaClO���� ��. NaClO����
��ͬѧ�ֱ�ȡ���������Һ���Թ�����ͨ������ʵ��ȷ���÷�Ӧ������һ�������������±�������֪������H2SO3>H2CO3>HClO��
ʵ����� | ʵ����� | ���� | ���� |
�� | ���뼸С��CaCO3���� | �����ݲ��� | ���� |
�� | �μ���������KI��Һ���� | __________ | �� |
�� | �μ�������ˮ���� | _________ | �� |
�� | �μ���������KMnO4��Һ���� | ��Һ����ɫ | ______ |
��5���ⶨij���Ѿ��п��������IJ�������������SO2�������ķ���������

(��֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2+I2 +2H2O==H2SO4+2HI)
������������ʵ�������ı�I2��Һ25.00mL���ô�ʵ������Ʒ�п��������IJ�������������SO2���㣩Ϊ_________g��L-1��
��������ʵ������������в���HI ���������������ý��________���ƫ�ߡ���ƫ�͡����䡱����