��Ŀ����
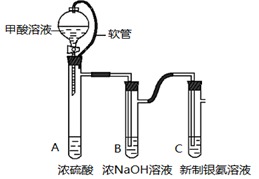
��12�֣�ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ��
��2���۲쵽A�е������� _______________________��
��3��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��д���йصĻ�ѧ����ʽ ��
��4��C��ʢ��CCl4�������� ��
��5����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м��� �������� ��
��1��C6H6+Br2 C6H5Br+HBr������2����ӦҺ�У��к���ɫ�������A������
C6H5Br+HBr������2����ӦҺ�У��к���ɫ�������A������
��3����ȥ�����屽���壬Br2+2NaOH=NaBr+NaBrO+H2O��2Br2+6NaOH=5NaBr+NaBrO3+3H2O����4����ȥ�廯�������е�����������5��ʯ����Һ����Һ���ɫ��
����

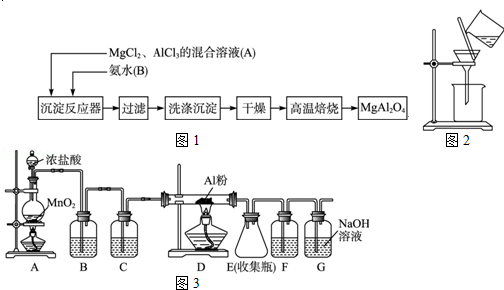
��ҵ�������̿���Ҫ�ɷ�ΪMnO2���Ʊ�������صĹ���������ͼ��ʾ��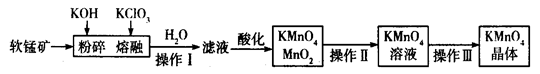
��1��������ص�������ǿ������Һ��������йأ��������������������Խ�ǿ������ ���������ữ���������Һ����____________������ţ���
| A������ | B��ϡ���� | C������ | D�������� |
��3�����̿����������KOH��KClO3������״̬�·�Ӧʱ����������__________________���ѧʽ������Ӧ���ˮ�ܽ�õ�����Һ����Ҫ����KCl��K2MnO4������Һ�ữʱ������Ӧ�����ӷ���ʽΪ _______________________________��
��4����֪KMnO4�����ȵľ������ữ��Na2C2O4��Ӧ����Mn2����CO2��ȡ�����Ƶõ�KMnO4��Ʒ0.33 g��ǡ����0.67 g Na2C2O4��ȫ��Ӧ����KMnO4�Ĵ���Ϊ________����
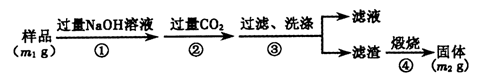
ʵ����ģ�����ij�Ͼɺ�����������Ҫ�ɷ�ΪNiO������Fe2O3��CaO��CuO��BaO�ȣ�����Ni2O3���乤������Ϊ��
��1������ͼ����ʾ��X��������ͼ�ף���֪����������������Ҫ�ɷ֣����С�����X��Ϊ ��ͼ���ʾ���Ľ��������¶ȵĹ�ϵ���������¶ȸ���70��ʱ�����Ľ����ʽ��ͣ���������Ni(OH)2�� ��������ԭ���� ��
��2�����������С�����Ʒ���Ļ�ѧʽΪ ��
��3����֪�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
| ��ʼ������pH | 1.5 | 6.5 | 7.7 |
| ������ȫ��pH | 3.7 | 9.7 | 9.2 |
����ԭ������ȷ����˵�����ɣ���ԭ������������Ը�������
��4������C��Ϊ�˳�ȥ��Һ�е�Ca2+����������Һ��F��Ũ��Ϊ3��10��3mol��L��1����Ca2+��Ũ��Ϊ mol��L��1��������ʱCaF2���ܶȻ�����Ϊ2.7��10��11��
��5��������2NiOOH��H2O��ԭ����������
�ټ���������Cl��������������ΪClO����
��Ni2+��ClO����������2NiOOH��H2O������
�ڢڲ���Ӧ�����ӷ���ʽΪ ��
��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�
NaBr + H2SO4 �� NaHSO4 + HBr
CH3CH2OH + HBr CH3CH2Br + H2O
CH3CH2Br + H2O
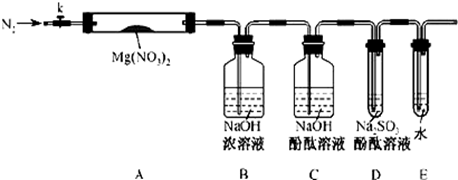
ij����С������ʵ�����Ʊ��������װ������ͼ���������±���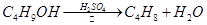
| ���� ���� | �Ҵ� | ������ | 1��2-�������� | ���� | Ũ���� |
| �ܶ�/g��cm-3 | 0.79 | 1.46 | 2.2 | 0.71 | 1.84 |
| �۵㣨�棩 | ��130 | ��119 | 9 | ��116 | 10 |
| �е㣨�棩 | 78.5 | 38.4 | 132 | 34.6 | 338 |
| ��ˮ�е��ܽ�ȣ�g/100gˮ�� | ���� | 0.914 | 1 | 7.5 | ���� |
��ش��������⡣
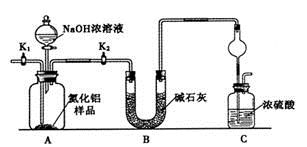
��1������ҩƷ֮ǰ�����IJ�����:_________________��ʵ����е�;��������δ�������Ƭ���䴦���ķ�����__________________��
��2��װ��B�������dz���ʹ���������������һ��Ŀ����_____________���¶ȼƵ��¶�Ӧ������_____________֮�䡣
��3����Ӧʱ�п�������SO2��һ�ֺ���ɫ���壬��ѡ������������Һ��ȥ�����壬�йص����ӷ���ʽ��___________��______________���˲�������___________����д�����������ƣ��н��У�ͬʱ���з��롣
��4��ʵ���в���80%���ᣬ��������98%Ũ���ᣬһ������Ϊ�˼��ٸ���Ӧ����һ������Ϊ��_______________________��
��5���ֲ�Ʒ�к��е���Ҫ�л�Һ��������_____________��Ϊ��һ���Ƶô����������飬�Դֲ�Ʒ����ˮϴ�ӡ���Һ���ټ�����ˮCaCl2������______________������