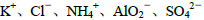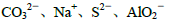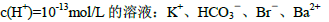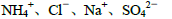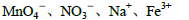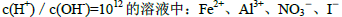��Ŀ����
ij��ѧ�о���ѧϰС��Ե������Һ�����µĹ����ܽ(���ڳ�����)
�ٳ����£�pH��1��ǿ����Һ����ˮϡ�ͺ���Һ�и����ӵ�Ũ��һ������
��pH��2��������pH��1�����ᣬc(H��)֮��Ϊ2��1
��25 ��ʱ��AgCl�����ڵ�����������ʵ���Ũ�ȵ�NaCl��CaCl2��Һ�е��ܽ�̶Ȳ�ͬ
��NH4HSO4��Һ�еμ�NaOH��Һ����ҺpH��7����c(Na��)��2c(SO42��)
����֪�������ƽ�ⳣ��ΪKa�������ˮ�ⳣ��ΪKh��ˮ�����ӻ�ΪKw�������߹�ϵΪ��Ka��Kh��Kw
�ס�������Һ����ǿ����ʣ���֪����ҺpH������ҺpH�����������������Һ�������ϣ����ҺpH���ܵ���7
�������ȷ����(����)
�ٳ����£�pH��1��ǿ����Һ����ˮϡ�ͺ���Һ�и����ӵ�Ũ��һ������
��pH��2��������pH��1�����ᣬc(H��)֮��Ϊ2��1
��25 ��ʱ��AgCl�����ڵ�����������ʵ���Ũ�ȵ�NaCl��CaCl2��Һ�е��ܽ�̶Ȳ�ͬ
��NH4HSO4��Һ�еμ�NaOH��Һ����ҺpH��7����c(Na��)��2c(SO42��)
����֪�������ƽ�ⳣ��ΪKa�������ˮ�ⳣ��ΪKh��ˮ�����ӻ�ΪKw�������߹�ϵΪ��Ka��Kh��Kw
�ס�������Һ����ǿ����ʣ���֪����ҺpH������ҺpH�����������������Һ�������ϣ����ҺpH���ܵ���7
�������ȷ����(����)
| A��ȫ�� | B���ۢݢ� | C���ܢݢ� | D���٢ڢ� |
��B
������c(OH��)������֮��Ϊ1��10������ΪCaCl2��Һ�е�c(Cl��)����NaCl��Һ�еģ�����AgCl(s) Ag��(aq)��Cl��(aq)����֪AgCl��NaCl��Һ�е��ܽ�̶ȴ���ȷ������Һ������ʱ��һ������NH4+�����ݵ���غ�c(Na��)��c(NH4+)��c(H��)��2c(SO42��)��c(OH��)��֪c(Na��)��c(NH4+)��2c(SO42��)����������CH3COOH??CH3COO����H����Ka��
Ag��(aq)��Cl��(aq)����֪AgCl��NaCl��Һ�е��ܽ�̶ȴ���ȷ������Һ������ʱ��һ������NH4+�����ݵ���غ�c(Na��)��c(NH4+)��c(H��)��2c(SO42��)��c(OH��)��֪c(Na��)��c(NH4+)��2c(SO42��)����������CH3COOH??CH3COO����H����Ka��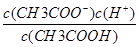 ��CH3COO����H2O??CH3COOH��OH����Kh��
��CH3COO����H2O??CH3COOH��OH����Kh�� ����֪Ka��Kh��c(H��)��c(OH��)����ȷ����������ҺpH��a������ҺpH��2a����Ϻ���Һ�����Լ�10��a��V(��)��102a��14��V(��)�����ԣ�a��2a��14����ʱa��14/3����ȷ��
����֪Ka��Kh��c(H��)��c(OH��)����ȷ����������ҺpH��a������ҺpH��2a����Ϻ���Һ�����Լ�10��a��V(��)��102a��14��V(��)�����ԣ�a��2a��14����ʱa��14/3����ȷ��
 Ag��(aq)��Cl��(aq)����֪AgCl��NaCl��Һ�е��ܽ�̶ȴ���ȷ������Һ������ʱ��һ������NH4+�����ݵ���غ�c(Na��)��c(NH4+)��c(H��)��2c(SO42��)��c(OH��)��֪c(Na��)��c(NH4+)��2c(SO42��)����������CH3COOH??CH3COO����H����Ka��
Ag��(aq)��Cl��(aq)����֪AgCl��NaCl��Һ�е��ܽ�̶ȴ���ȷ������Һ������ʱ��һ������NH4+�����ݵ���غ�c(Na��)��c(NH4+)��c(H��)��2c(SO42��)��c(OH��)��֪c(Na��)��c(NH4+)��2c(SO42��)����������CH3COOH??CH3COO����H����Ka��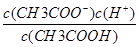 ��CH3COO����H2O??CH3COOH��OH����Kh��
��CH3COO����H2O??CH3COOH��OH����Kh�� ����֪Ka��Kh��c(H��)��c(OH��)����ȷ����������ҺpH��a������ҺpH��2a����Ϻ���Һ�����Լ�10��a��V(��)��102a��14��V(��)�����ԣ�a��2a��14����ʱa��14/3����ȷ��
����֪Ka��Kh��c(H��)��c(OH��)����ȷ����������ҺpH��a������ҺpH��2a����Ϻ���Һ�����Լ�10��a��V(��)��102a��14��V(��)�����ԣ�a��2a��14����ʱa��14/3����ȷ��
��ϰ��ϵ�д�
�����Ŀ
 ��ClO-
��ClO-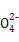 ��SCN-
��SCN- =1012����Һ��:N
=1012����Һ��:N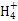 ��Al3+��S
��Al3+��S