��Ŀ����
�����£������й���Һ��pH���������ʵ���Ũ�ȹ�ϵ���жϲ���ȷ����(����)��
A��pH��3�Ĵ�����Һ��pH��11��NaOH��Һ�������ϣ�������Һ��pH<7
B��pH��3�Ķ�Ԫ����H2R��Һ��pH��11��NaOH��Һ��Ϻ����Һ��pH��7����Ӧ��Ļ����Һ�У�2c(R2��)��c(HR��)��c(Na��)
C����0.2 mol��L��1��ijһԪ��HA��Һ��0.1 mol��L��1 NaOH��Һ�������Ϻ����Һ��pH����7����Ӧ��Ļ����Һ�У�2c(OH��)��2c(H��)��c(HA)��c(A��)
D��ij���ʵ���Һ����ˮ�������c(H��)��1��10��a mol��L��1����a>7�������Һ��pHһ��Ϊ14��a
A��pH��3�Ĵ�����Һ��pH��11��NaOH��Һ�������ϣ�������Һ��pH<7
B��pH��3�Ķ�Ԫ����H2R��Һ��pH��11��NaOH��Һ��Ϻ����Һ��pH��7����Ӧ��Ļ����Һ�У�2c(R2��)��c(HR��)��c(Na��)
C����0.2 mol��L��1��ijһԪ��HA��Һ��0.1 mol��L��1 NaOH��Һ�������Ϻ����Һ��pH����7����Ӧ��Ļ����Һ�У�2c(OH��)��2c(H��)��c(HA)��c(A��)
D��ij���ʵ���Һ����ˮ�������c(H��)��1��10��a mol��L��1����a>7�������Һ��pHһ��Ϊ14��a
��D
��ѡ��A������ԶԶ������������Һ�����ԣ�A��ȷ��ѡ��B�����ݵ���غ��ж�����ȷ�ġ�ѡ��C���ɵ���غ�ã�c(OH��)��c(A��)��c(H��)��
c(Na��)���������غ�ã�2c(Na��)��c(A��)��c(HA)����ʽ��Ͽɵ�2c(OH��)��
2c(H��)��c(HA)��c(A��)��ѡ��D��˵��ˮ�ĵ��뱻���ƣ�������������Һ��Ҳ�����Ǽ�����Һ�������Һ��pH����a��14��a��
c(Na��)���������غ�ã�2c(Na��)��c(A��)��c(HA)����ʽ��Ͽɵ�2c(OH��)��
2c(H��)��c(HA)��c(A��)��ѡ��D��˵��ˮ�ĵ��뱻���ƣ�������������Һ��Ҳ�����Ǽ�����Һ�������Һ��pH����a��14��a��

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ
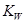 �����¶ȵ����߶�����˵��ˮ�ĵ����Ƿ��ȷ�Ӧ
�����¶ȵ����߶�����˵��ˮ�ĵ����Ƿ��ȷ�Ӧ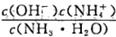 ����
����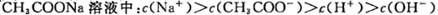
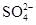 ��Cl-
��Cl-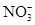
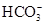 ��Cl-
��Cl-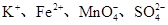

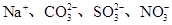
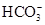 ����Һ��
����Һ��
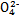 ��S
��S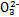 ��Si
��Si
 H++OH-��KW=10-14
H++OH-��KW=10-14 b(����ڡ���С�ڡ����ڡ�)����a��b��ʾNH3��H2O�ĵ���ƽ�ⳣ��Ϊ����������
b(����ڡ���С�ڡ����ڡ�)����a��b��ʾNH3��H2O�ĵ���ƽ�ⳣ��Ϊ����������  ��Br����Ba2+
��Br����Ba2+