��Ŀ����
��a mL������Һ�еμ�0.01 mol��L��1������������Һ���ζ�������ͼ��ʾ��
(1)������ҺŨ��________(����ڡ���С�ڡ����ڡ�)0.01 mol��L��1��������________��
(2)b�㣬c(Na��)________c(CH3COO��)(�>����<������)
(3)������������������Һǡ����ȫ�к�ʱ�������϶�Ӧ�ĵ�QӦ��____��
A��2��a֮�� B��a��b֮��
C��b��c֮�� D��a��c֮��
(4)���й�ϵʽһ����ȷ����________��
A��a�㣬c(H��)>c(OH��)>c(CH3COO��)>c(Na��)
B��a�㣬c(Na��)>c(CH3COO��)>c(H��)>c(OH��)
C��c�㣬c(Na��)>c(CH3COO��)>c(OH��)>c(H��)
D��c�㣬c(Na��)��c(H��)��c(CH3COO��)��c(OH��)
(1)���ڣ�CH3COOH��������ʣ�ֻ���ֵ���
(2)����(3)C��(4)D
����

 ��У����ϵ�д�
��У����ϵ�д�ijѧ����0.100 mol��L-1��KOH����Һ�ζ�δ֪Ũ�ȵ�����,������ɷֽ�Ϊ���¼���:
| A����ȡ20.00 mL����������Һע��ྻ����ƿ,������2~3�η�̪; |
| B���ñ���Һ��ϴ�ζ���2~3��; |
| C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ���,���ڵζ��ܼ���ʹ֮������Һ; |
| D��ȡ��KOH��Һע���ʽ�ζ������̶ȡ�0������2~3 mL; |
F.����ƿ���ڵζ��ܵ�����,�ñ�KOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȡ�
�ʹ�ʵ��������:
(1)��ȷ���������˳����(����ĸ�����д)��������������������
(2)����B���������Ŀ������������������������������������������
(3)����A�������֮ǰ,�����ô�����Һ��ϴ��ƿ,��ζ��������������(�ƫ�ߡ���ƫ�͡����䡱)��
(4)�жϵ���ζ��յ��ʵ����������������������������������������������
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�
��֪��Zn���仯�����������Al���仯������������ƣ���ش��������⣺
(1)��NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
(3)����ҺB��ȡFe3O4�������ӵĹ����У������ͨ��N2����ԭ����
_______________________________________________________________
(4)Fe3O4���������ܷ��ü�ѹ���˷�ʵ�ֹ�Һ���룿__________(��ܡ����ܡ�)��������________________________________��
(5)���ظ���ط�(һ��������ԭ�ζ���)�ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.010 00 mol��L��1��K2Cr2O7����Һ250 mL��Ӧȷ��ȡ________ g K2Cr2O7(����4λ��Ч���֣���֪MK2Cr2O7��294.0 g��mol��1)�����Ƹñ���Һʱ�����������в���Ҫ�õ�����________(�ñ�ű�ʾ)��
�ٵ�����ƽ�����ձ�������Ͳ���ܲ�����
������ƿ ��ͷ�ιܡ�����Һ��
(6) �ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ⶨ�����________(�ƫ����ƫС�����䡱)��
25 ��ʱ����Ũ��Ϊ0.100 0 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.100 0 mol��L��1��������HX��HY��HZ���ζ�������ͼ��ʾ������˵����ȷ���� (����)��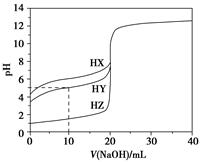
| A������ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX |
| B�����ݵζ����ߣ��ɵ�Ka(HY)��10��5 |
| C��������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��c(X��)��c(Y��)��c(OH��)��c(H��) |
D��HY��HZ��ϣ��ﵽƽ��ʱc(H��)�� ��c(Z��)��c(OH��) ��c(Z��)��c(OH��) |
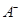 ������Ŀ�仯���Ƶ��� (����ĸ)��
������Ŀ�仯���Ƶ��� (����ĸ)��
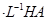 ��Һ��1���0.02mol
��Һ��1���0.02mol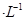 NaOH��Һ��ϣ��õ�2��������Һ��
NaOH��Һ��ϣ��õ�2��������Һ�� 0��01 mol
0��01 mol
 ��
��