��Ŀ����
��������dz��õ��������������ǹ�ҵ�������̿��Ʊ�������ص�һ�ֹ������̣�
��1��������ر�������ɫ�Լ�ƿ�������Լ����治��Ҫ��ɫ�Լ�ƿ����______ ������ţ�
a��Ũ���� b�������� c����ˮ d���ռ�
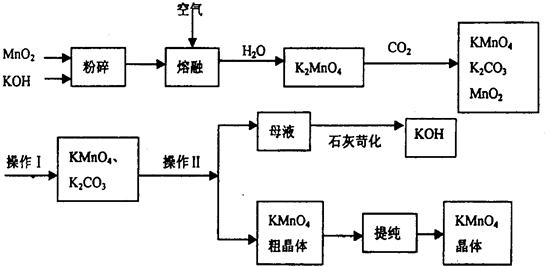
��2��K2MnO4��C02��Ӧ����KMnO4��MnO2��K2C03�Ļ�ѧ����ʽΪ��______
��3�����������п�ѭ��ʹ�õ�������______ ���û�ѧʽ��ʾ������д2�֣���
��4������I��������______���ýᾧ������KMnO4��K2C03�������ʣ����иò���ǰ����ĵ�������______��
��5��������KMnO4���ܽ�����ݣ�
ʵ�������ؽᾧ�ķ�����KMnO4��Ʒ�Ƴɾ�Ʒ������еIJ����У������ȱ�����Һ��______�����ˡ�ϴ�ӡ�______��ϴ������Ҫ�IJ����������ձ�����������______������Ƿ�ϴ���IJ�����______��

��1��������ر�������ɫ�Լ�ƿ�������Լ����治��Ҫ��ɫ�Լ�ƿ����______ ������ţ�
a��Ũ���� b�������� c����ˮ d���ռ�
��2��K2MnO4��C02��Ӧ����KMnO4��MnO2��K2C03�Ļ�ѧ����ʽΪ��______
��3�����������п�ѭ��ʹ�õ�������______ ���û�ѧʽ��ʾ������д2�֣���
��4������I��������______���ýᾧ������KMnO4��K2C03�������ʣ����иò���ǰ����ĵ�������______��
��5��������KMnO4���ܽ�����ݣ�
| �¶ȣ��棩 | O | 10 | 20 | 50 | 60 |
| S��g/lOOgˮ�� | 2.8 | 4.3 | 6.3 | 17.0 | 22.1 |
��1��Ũ���ᡢ����������ˮ����ֽ���Ҫ��������ɫ�Լ�ƿ�У�KOH����Ҫ��������ɫ�Լ�ƿ�У�
��ѡ��d��
��2����Ӧ��K2MnO4��KMnO4��MnԪ�ػ��ϼ���+6������Ϊ+7�ۣ������߱仯1�ۣ�K2MnO4��MnO2��MnԪ�ػ��ϼ���+6�۽���Ϊ+4�ۣ�������2�ۣ����ϼ�������С������Ϊ2����KMnO4ϵ��Ϊ2��MnO2ϵ��Ϊ1������MnԪ���غ��֪KMnO4ϵ��Ϊ3��
����KԪ���غ��֪K2C03ϵ��Ϊ2����̼Ԫ���غ��֪ϵ��C02Ϊ2������ʽΪ3K2MnO4+2CO2=2KMnO4+2K2CO3+MnO2��
�ʴ�Ϊ��3K2MnO4+2CO2=2KMnO4+2K2CO3+MnO2��
��3����ת����ϵͼ֪KOH��MnO2���Ա�ѭ�����ã�
�ʴ�Ϊ��KOH��MnO2��
��4�����˳�ȥ������ˮ��MnO2�������ܽ�ȵIJ�ͬ����ȡŨ���ᾧ�ķ���ʹKMnO4����Һ�����������������
�ʴ�Ϊ�����ˣ�KMnO4��K2C03���ܽ�����¶ȱ仯�����
��5��ʵ�������ؽᾧ�ķ�����KMnO4��Ʒ�Ƴɾ�Ʒ��Ӧ�����ȱ�����Һ����ȴ���塢���ˡ�ϴ�ӡ�������˽�������ϴ�ӣ�ϴ������Ҫ�IJ����������ձ�����������©������������Ҫ��������K2CO3������ȡ�ڶ���ϴ��Һ������CaCl2��Һ����û�а�ɫ�������ɣ�˵��ϴ�Ӹɾ���
�ʴ�Ϊ����ȴ���壻 ���©����ȡ�ڶ���ϴ��Һ������CaCl2��Һ����û�а�ɫ�������ɣ�˵��ϴ�Ӹɾ���
��ѡ��d��
��2����Ӧ��K2MnO4��KMnO4��MnԪ�ػ��ϼ���+6������Ϊ+7�ۣ������߱仯1�ۣ�K2MnO4��MnO2��MnԪ�ػ��ϼ���+6�۽���Ϊ+4�ۣ�������2�ۣ����ϼ�������С������Ϊ2����KMnO4ϵ��Ϊ2��MnO2ϵ��Ϊ1������MnԪ���غ��֪KMnO4ϵ��Ϊ3��
����KԪ���غ��֪K2C03ϵ��Ϊ2����̼Ԫ���غ��֪ϵ��C02Ϊ2������ʽΪ3K2MnO4+2CO2=2KMnO4+2K2CO3+MnO2��
�ʴ�Ϊ��3K2MnO4+2CO2=2KMnO4+2K2CO3+MnO2��
��3����ת����ϵͼ֪KOH��MnO2���Ա�ѭ�����ã�
�ʴ�Ϊ��KOH��MnO2��
��4�����˳�ȥ������ˮ��MnO2�������ܽ�ȵIJ�ͬ����ȡŨ���ᾧ�ķ���ʹKMnO4����Һ�����������������
�ʴ�Ϊ�����ˣ�KMnO4��K2C03���ܽ�����¶ȱ仯�����
��5��ʵ�������ؽᾧ�ķ�����KMnO4��Ʒ�Ƴɾ�Ʒ��Ӧ�����ȱ�����Һ����ȴ���塢���ˡ�ϴ�ӡ�������˽�������ϴ�ӣ�ϴ������Ҫ�IJ����������ձ�����������©������������Ҫ��������K2CO3������ȡ�ڶ���ϴ��Һ������CaCl2��Һ����û�а�ɫ�������ɣ�˵��ϴ�Ӹɾ���
�ʴ�Ϊ����ȴ���壻 ���©����ȡ�ڶ���ϴ��Һ������CaCl2��Һ����û�а�ɫ�������ɣ�˵��ϴ�Ӹɾ���

��ϰ��ϵ�д�
�����Ŀ
