题目内容
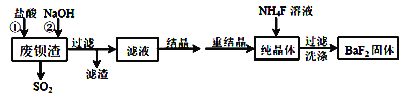
【题目】利用废钡渣(主要成分为BaS2O3,含少量SiO2)为原料生产高纯氟化钡的流程如下:
已知:Ksp(BaS2O3)=6.96×10-11,Ksp(BaF2)=1.0×10-6
(1)加入盐酸时除产生SO2外,还有淡黄色固体生成。该反应的离子方程式为___________________________________________________________。
(2)滤液的主要成分有____________。(填化学式)
(3)工业上可用氨水吸收SO2,并通入空气使其转化为铵态氮肥。该转化中氧化剂与还原剂的物质的量之比为_____________。
(4)加入NaOH溶液的目的是中和过量的盐酸,但不宜过量,其原因是____________(用离子反应方程式表示)。
(5)生成BaF2的反应的化学方程式为_________________________。
①若该反应温度过高,容易造成c(F-)降低的原因是_______________________。
②研究表明,适当增加NH4F的比例有利于提高BaF2的产率和纯度。将浓度为0.1 molL-1的BaCl2溶液和0.22molL-1NH4F溶液等体积混合,所得溶液中c(Ba2+)=__________。
【答案】BaS2O3+2H+=Ba2++S↓+SO2↑+H2O BaCl2、NaCl 1︰2 2OH-+SiO2=SiO32-+H2O BaCl2+2NH4F=BaF2↓+2NH4Cl 温度较高促进F-水解,使c(F-)降低 0.01 molL-1
【解析】
根据流程图中的产物和反应物分析涉及的反应;根据得失电子总数相等分析氧化剂与还原剂的物质的量之比,根据溶度积计算溶液中的离子浓度。
(1)加入盐酸时除产生SO2外,还有淡黄色固体生成,则该反应的离子方程式为:BaS2O3+2H+=Ba2++S↓+SO2↑+H2O;
(2)滤液的主要成分是盐酸与BaS2O3反应所得的可溶性产物:BaCl2、NaCl;
(3)工业上可用氨水吸收SO2,并通入空气使其转化为铵态氮肥,反应中还原剂为二氧化硫,氧化剂为氧气,S元素化合价升高2,氧化合价降低2,故该转化中氧化剂与还原剂的物质的量之比为:1:2;
(4)二氧化硅属于酸性氧化物,能与碱反应,离子方程式为:2OH-+SiO2=SiO32-+H2O ;(5)溶液中含有氯化钡,与NH4F反应生成BaF2的化学方程式为:BaCl2+2NH4F=BaF2↓+2NH4Cl ;
①若该反应温度过高,容易造成c(F-)降低的原因是:温度较高促进F-水解,使c(F-)降低;
②Ksp(BaF2)= c2(F-)×c(Ba2+)=1.0×10-6,c(F-)=c(NH4F)/2=0.22molL-1/2=0.11 molL-1,则 c(Ba2+)= 0.01 molL-1。

 阅读快车系列答案
阅读快车系列答案