��Ŀ����
����Ŀ����1��ú�����ƺϳ���(CO��H2)
��֪��C(s)��H2O(g)===CO(g)��H2(g)��H2��131.3kJ��mol1
C(s)��2H2O(g)===CO2(g)��2H2(g)��H2��90kJ��mol1
��һ����̼��ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ��_____
��2���ɺϳ����Ƽ״�
�ϳ���CO��H2��һ���������ܷ�����Ӧ��CO(g)��2H2(g)![]() CH3OH(g)��H��0��
CH3OH(g)��H��0��
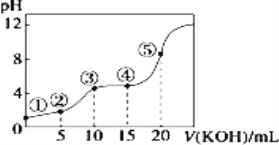
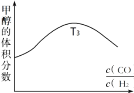
�����ݻ���ΪVL�ļס��ҡ��������ĸ��ܱ������зֱ����amolCO��2amolH2���ĸ������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3��T4�Һ㶨���䡣������������ͬ������£�ʵ���÷�Ӧ���е�tminʱH2�����������ͼ��ʾ����T3�¶��µĻ�ѧƽ�ⳣ��Ϊ_____(��a��V��ʾ)
��ͼ��ӳ������T3�¶��£���Ӧ����tmin��״�����������뷴Ӧ���ʼͶ�ϱ�![]() �Ĺ�ϵ���뻭��T4�¶��µı仯�������ߡ�______________
�Ĺ�ϵ���뻭��T4�¶��µı仯�������ߡ�______________
����ʵ�ʹ�ҵ�����У�Ϊ�ⶨ���º�ѹ�����·�Ӧ�Ƿ�ﵽƽ��״̬������Ϊ�ж����ݵ���_____
A�������������ܶȱ��ֲ��� B��CO ������������ֲ���
C�������ƽ����Է����������ֲ��� D��c(H2)=2c(CH3OH)
��3���ɼ״���ϩ��
����Ӧ��2CH3OH![]() C2H4��2H2O i��
C2H4��2H2O i��
3CH3OH![]() C3H6��3H2O ii
C3H6��3H2O ii
����Ӧ��2CH3OH![]() CH3OCH3��H2O iii
CH3OCH3��H2O iii
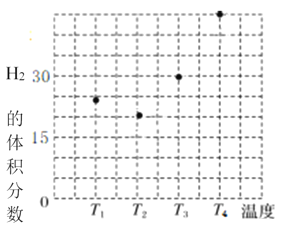
ijʵ���ҿ��Ʒ�Ӧ�¶�Ϊ400��������ͬ�ķ�Ӧ��ϵ�зֱ���װ���������ִ���(Cat.1��Cat.2)���Ժ㶨������ͨ��CH3OH������ͬ��ѹǿ�½��м״���ϩ���ĶԱ��о����õ�����ʵ������(ѡ���ԣ�ת���ļ״���������ϩ�ͱ�ϩ�İٷֱ�)
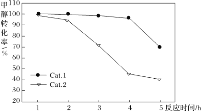
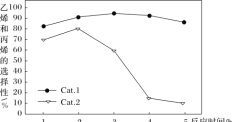
��ͼ���֪��ʹ��Cat.2��Ӧ2h��״���ת��������ϩ�ͱ�ϩ��ѡ���Ծ������½������ܵ�ԭ���ǣ������ײ���۽��ͣ�_____
���𰸡�CO(g)+H2O(g)=CO2(g)+H2(g) ��H=-41.3 kJ��mol-1 539V2/27a2  ABC ��������2h�����ʧ��״�ת���ʽϵͣ�Cat.2�������ͷ�Ӧiii�Ļ�ܣ������Ӱٷ�������ͬʱ���ڿ������ɸ���������ѣ�Ŀ�����ѡ�����½�
ABC ��������2h�����ʧ��״�ת���ʽϵͣ�Cat.2�������ͷ�Ӧiii�Ļ�ܣ������Ӱٷ�������ͬʱ���ڿ������ɸ���������ѣ�Ŀ�����ѡ�����½�
��������
�Ÿ��ݸ�˹���ɵõ�һ����̼��ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ��
������������ʽ����ƽ��ʱ�����ʵ������ټ���ƽ�ⳣ����
���÷�Ӧ�Ƿ��ȷ�Ӧ������ͼ�ó���Ӧ��tminʱ��T2�¶�H2�����������ͣ�T3��T4�¶ȸߣ�H2����������ߣ�˵��ƽ�������ƶ�������ͼ�ó�T4�¶ȼ״��ı仯�������ߣ�
��A�����º�ѹ�����������������ܶȵ�����������������������������������䣬���������С���ܶ�������˿���Ϊ�ж�ƽ��ı�־��
B����ʼ��CO������������ϼ�С��������������䣬�����Ϊ�ж�ƽ��ı�־��
C�������ƽ����Է�������������������������������ʵ����������������䣬��������ʵ�����С�������ƽ����Է��������������ֲ������Ϊ�ж�ƽ��ı�־��
D��c(H2) = 2c(CH3OH)��������Ũ�ȱ������ж�ƽ��ı�־��
����ͼ���֪��ʹ��Cat.2��Ӧ2h��״���ת��������ϩ�ͱ�ϩ��ѡ���Ծ������½������ܵ�ԭ���ǣ����ݵ�һ��ͼ�ó���������2h�����ʧ��״�ת���ʽϵͣ��ڶ���ͼ�ó�Cat.2�������ͷ�Ӧiii�Ļ�ܣ������Ӱٷ�������ͬʱ���ڿ������ɸ���������ѣ�������ϩ����ϩĿ�����ѡ�����½���
�Ž���2������ʽ��ȥ��1������ʽ���õ�һ����̼��ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ��CO(g)+H2O=CO2(g)+H2(g) ��H=41.3 kJ��mol1
����
![]() �����
�����![]() ����
���� ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
���÷�Ӧ�Ƿ��ȷ�Ӧ������ͼ�ó���Ӧ��tminʱ��T2�¶�H2�����������ͣ�T3��T4�¶ȸߣ�H2����������ߣ�˵��ƽ�������ƶ���ͼ��ӳ������T3�¶��£���Ӧ����tmin��״�����������뷴Ӧ���ʼͶ�ϱ�![]() �Ĺ�ϵ����T4�¶ȼ״�������T3�¶ȼ״�����С�����仯�������ߡ�
�Ĺ�ϵ����T4�¶ȼ״�������T3�¶ȼ״�����С�����仯�������ߡ� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��A�����º�ѹ�����������������ܶȵ�����������������������������������䣬���������С���ܶ�������˿���Ϊ�ж�ƽ��ı�־����A�������⣻
B����ʼ��CO������������ϼ�С��������������䣬�����Ϊ�ж�ƽ��ı�־����B�������⣻
C�������ƽ����Է�������������������������������ʵ����������������䣬��������ʵ�����С�������ƽ����Է��������������ֲ������Ϊ�ж�ƽ��ı�־����C�������⣻
D��c(H2) = 2c(CH3OH)��������Ũ�ȱ������ж�ƽ��ı�־����D���������⣻
��������������ABC��
����ͼ���֪��ʹ��Cat.2��Ӧ2h��״���ת��������ϩ�ͱ�ϩ��ѡ���Ծ������½������ܵ�ԭ���ǣ����ݵ�һ��ͼ�ó���������2h�����ʧ��״�ת���ʽϵͣ��ڶ���ͼ�ó�Cat.2�������ͷ�Ӧiii�Ļ�ܣ������Ӱٷ�������ͬʱ���ڿ������ɸ���������ѣ�������ϩ����ϩĿ�����ѡ�����½����ʴ�Ϊ����������2h�����ʧ��״�ת���ʽϵͣ�Cat.2�������ͷ�Ӧiii�Ļ�ܣ������Ӱٷ�������ͬʱ���ڿ������ɸ���������ѣ�Ŀ�����ѡ�����½���

����Ŀ���Ȼ���ͭ��![]() ���������л��ϳɹ�ҵ�еĴ�������һ�ְ�ɫ��ĩ������ˮ���������Ҵ���ϡ���ᡣ��ҵ��������ӡˢ��·�ķ�Һ����
���������л��ϳɹ�ҵ�еĴ�������һ�ְ�ɫ��ĩ������ˮ���������Ҵ���ϡ���ᡣ��ҵ��������ӡˢ��·�ķ�Һ����![]() ��
��![]() ��
��![]() ��
��![]() ������
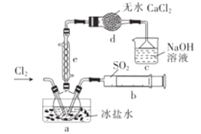
������![]() ��������ͼ��ʾ��
��������ͼ��ʾ��
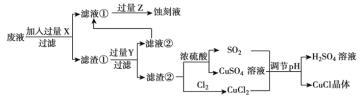
����������Ϣ�ش��������⣺
��1�����������У�X��________��Z��________�������ѧʽ��
��2��д������![]() �����ӷ���ʽ________��
�����ӷ���ʽ________��
��3��������![]() ���岻��ˮ������ˮ�Ҵ�ϴ�ӵ�ԭ����________��
���岻��ˮ������ˮ�Ҵ�ϴ�ӵ�ԭ����________��
��4����![]() �����ɹ����������ϲ���Ҫ����SO2���壬��������________��
�����ɹ����������ϲ���Ҫ����SO2���壬��������________��
��5����֪��������![]() ��
��![]() ������
������![]() ������Һ�м���
������Һ�м���![]() ������
������![]() ����ʱ��Һ��
����ʱ��Һ��![]() =________��
=________��
��6��ʵ��̽��pH��![]() ���ʵ�Ӱ�����±���ʾ��
���ʵ�Ӱ�����±���ʾ��
pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 70 | 90 | 82 | 78 | 75 | 72 | 70 |
����![]() �������pHΪ________����pH�ϴ�ʱ
�������pHΪ________����pH�ϴ�ʱ![]() ���ʱ�͵�ԭ����________��
���ʱ�͵�ԭ����________��