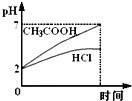
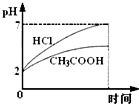
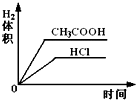
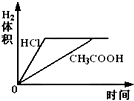
��Ŀ����
4���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��û�ѧ����ش��������⣺���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| һ | �� | |||||||
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� |
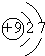 ����Ԫ�ص������ǹ裬��Ԫ�ص����ӵĵ���ʽ��
����Ԫ�ص������ǹ裬��Ԫ�ص����ӵĵ���ʽ��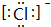 ���ڵ������̬�⻯���еĻ�ѧ���м��Լ�������Ӽ����������Լ����Ǽ��Լ�������
���ڵ������̬�⻯���еĻ�ѧ���м��Լ�������Ӽ����������Լ����Ǽ��Լ���������2���ڱ��Ԫ���У�����õĽ���Ԫ���ǣ�дԪ�ط��ţ�Na������õķǽ���Ԫ���ǣ�дԪ�ط��ţ�F��
��3���ߡ��ࡢ��Ԫ�����γɵ���̬�⻯���У����ȶ����ǣ�д��ѧʽ��HCl��
��4���ݡ��ޡ�������Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳���ǣ�дԪ�ط��ţ�Na��Al��Si��
��5��������γɹ��ۻ�����ĵ���ʽ
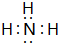 ���õ���ʽ��ʾ������γɻ�������γɹ���
���õ���ʽ��ʾ������γɻ�������γɹ���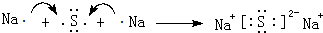 ��
����6���ߡ��ࡢ������Ԫ�ص���ۺ������������ǿ������˳���ǣ�д��ѧʽ��HClO4��H2SO4��H2SiO3��
���� ��Ԫ�������ڱ��е�λ�ã���֪��ΪH����ΪC����ΪN����ΪF����ΪNa����ΪAl����ΪSi����ΪS����ΪCl��
��1����ΪF��ԭ�Ӻ�����2�����Ӳ㣬���������Ϊ2��7����Ϊ��Ԫ�أ���Ԫ�ص�����ΪCl-������������Ϊ8����һ����λ����ɣ��ڵ������̬�⻯��ΪCH4��
��2��ͬ����������ҽ����Լ������ǽ�������ǿ��ͬ�������϶��½�������ǿ���ǽ����Լ�����
��3���ǽ�����Խǿ���⻯��Խ�ȶ���
��4��ͬ�����������ԭ�Ӱ뾶��С��
��5��������γɹ��ۻ�����ΪNH3��������Nԭ����Hԭ��֮���γ�1�Թ��õ��Ӷԣ�
������γɻ�����ΪNa2S�����������������ӹ��ɣ�
��6���ǽ�����Խǿ����Ӧ����������������Խǿ��
��� �⣺��Ԫ�������ڱ��е�λ�ã���֪��ΪH����ΪC����ΪN����ΪF����ΪNa����ΪAl����ΪSi����ΪS����ΪCl��
��1����ΪF��ԭ�Ӻ�����2�����Ӳ㣬���������Ϊ2��7��ԭ�ӽṹʾ��ͼΪ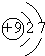 ����Ϊ��Ԫ�أ���Ԫ�ص�����ΪCl-������������Ϊ8����һ����λ����ɣ�����ʽΪ
����Ϊ��Ԫ�أ���Ԫ�ص�����ΪCl-������������Ϊ8����һ����λ����ɣ�����ʽΪ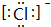 ���ڵ������̬�⻯��ΪCH4��C��Hԭ��֮���γɼ��Լ���
���ڵ������̬�⻯��ΪCH4��C��Hԭ��֮���γɼ��Լ���
�ʴ�Ϊ��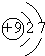 ���裻
���裻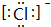 �����Լ���
�����Լ���
��2��ͬ����������ҽ����Լ������ǽ�������ǿ��ͬ�������϶��½�������ǿ���ǽ����Լ�����������Ԫ��������õĽ���Ԫ����Na������õķǽ���Ԫ����F��
�ʴ�Ϊ��Na��F��
��3���ǽ�����Cl��S��Si���ǽ�����Խǿ���⻯��Խ�ȶ�����HCl���ȶ����ʴ�Ϊ��HCl��
��4��ͬ�����������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��Na��Al��Si���ʴ�Ϊ��Na��Al��Si��
��5��������γɹ��ۻ�����ΪNH3��������Nԭ����Hԭ��֮���γ�1�Թ��õ��Ӷԣ�����ʽΪ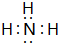 ��
��
������γɻ�����ΪNa2S�����������������ӹ��ɣ��õ���ʽ��ʾ�γɹ���Ϊ��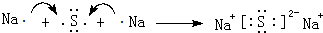 ��
��
�ʴ�Ϊ��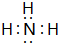 ��
��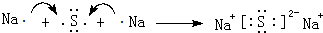 ��
��
��6���ǽ�����Cl��S��Si���ǽ�����Խǿ����Ӧ����������������Խǿ�������ԣ�HClO4��H2SO4��H2SiO3���ʴ�Ϊ��HClO4��H2SO4��H2SiO3��
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ�Ӧ�ã��ѶȲ�������Ԫ�������ɵ�Ӧ�ã�ѧϰ��ע��������֪ʶ��ע�����յ���ʽ��ʾ��ѧ�������ʵ��γɣ�

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�| A�� | dcab | B�� | dabc | C�� | dbac | D�� | badc |
����С�������ѹǿ�ڽ����¶Ȣ�����COŨ�Ȣ�����̼������
| A�� | �٢ڢ� | B�� | �٢� | C�� | �٢ڢۢ� | D�� | �ۢ� |
| A�� | ������Ϊ17��������Ϊ20����ԭ�ӣ�${\;}_{17}^{37}$Cl | |
| B�� | �����ӵĽṹʾ��ͼ�� | |
| C�� | ������ӵı���ģ�ͣ� | |
| D�� | ����ϩ���ӵĽṹ��ʽ��CH2-CH2Cl |
| A�� | 65%�ľƾ��������� | B�� | Al��OH��3����������θ����� | ||
| C�� | ��������ˮ��ɱ������ | D�� | �Ӻ�ˮ����ȡ��ȼ�� |
| A�� | Cl2ͨ��ˮ�У�Cl2+H2O�T2H++Cl-+ClO- | |
| B�� | ��������Һ�еμ�Ba��OH��2��Һ��ǡ��ʹSO42-������ȫ��2Al3++3SO42-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4�� | |
| C�� | ��CH3COOH�ܽ�CaCO3��CaCO3+2H+�TCa2++H2O+CO2�� | |
| D�� | Ca��HCO3��2��Һ������NaOH��Һ��Ӧ��HCO3-+Ca2++OH-�TCaCO3��+H2O |