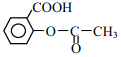��Ŀ����
����Ŀ�������Թ�ѧ��������Ϣ�����⼼����ҽ�ơ����������������ҪӦ�ü�ֵ���ҹ���ѧ������Cs2CO3��XO2��X��Si��Ge����H3BO3�״κϳ������ΪCsXB3O7�ķ����Թ�ѧ���塣�ش��������⣺
��1��C��O��Si����Ԫ�ص縺���ɴ�С��˳��Ϊ__________����һ������I1(Si) ____ I1(Ge)����������������������
��2����̬Geԭ�Ӻ�������Ų�ʽΪ_____________��SiO2��GeO2�������Ƶľ���ṹ�������۵�ϸߵ���________��ԭ����______��
��3����ͼΪH3BO3�����Ƭ��ṹ������B���ӻ���ʽΪ________����������ˮ�б�����ˮ���ܽ�������������Ҫԭ����_____________��
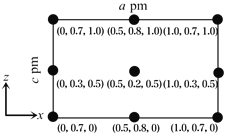
��4���Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������ꡣCsSiB3O7����������ϵ���������Σ�����������Ϊa pm��b pm��c pm����ͼΪ��y��ͶӰ�ľ���������Csԭ�ӵķֲ�ͼ��ԭ�ӷ������ꡣ�ݴ��ƶϸþ�����Csԭ�ӵ���ĿΪ_______��CsSiB3O7��Ħ������ΪM g��mol��1����NAΪ�����ӵ�������ֵ����CsSiB3O7������ܶ�Ϊ_____________g��cm��1���ô���ʽ��ʾ����
���𰸡�O��C��Si �� 1s22s22p63s23p63d104s24p2����[Ar]3d104s24p2�� SiO2 ���߾�Ϊԭ�Ӿ��壬Geԭ�Ӱ뾶����Si��Si��O����С��Ge��O������SiO2���ܸ����۵���ߡ� sp2 ��ˮ�ƻ������ᾧ���е�������������������ˮ�γɷ��Ӽ������ʹ�ܽ������ 4 ![]()
��������
��1���縺�Եı仯����Ϊͬ���ڴ�������������ͬ���������������٣���һ�����ܵı仯����Ϊͬ��Ԫ������������С��
��2��SiO2��GeO2Ϊͬ���;���ṹ����Ϊԭ�Ӿ��壬Geԭ�Ӱ뾶����Si��Si-O����С��Ge-O������SiO2���ܸ����۵���ߡ�
��3��Bԭ���������3�����ӣ���3��-OH�γ�3�����ۼ������ɵ��ӻ���ʽ����ˮ�ƻ������ᾧ���е�������������������ˮ�γɷ��Ӽ������ʹ�ܽ������
��4���ȷ����ó�Cs�ĸ������ٸ��ݹ�ʽ�����ܶȡ�
��1���縺�Եı仯����Ϊͬ���ڴ�������������ͬ���������������٣����Ե縺��O>C>Si����һ�����ܵı仯����Ϊͬ��Ԫ������������С�����I1(Si)>I1(Ge),
�ʴ�ΪO��C��Si��>��
��2��Geԭ��λ�ڵ�������IVA�壬���ԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d104s24p2����[Ar]3d104s24p2����SiO2��GeO2��Ϊԭ�Ӿ��壬Geԭ�Ӱ뾶����Si��Si-O����С��Ge-O������SiO2���ܸ����۵���ߣ�
�ʴ�Ϊ1s22s22p63s23p63d104s24p2����[Ar]3d104s24p2����SiO2�����߾�Ϊԭ�Ӿ��壬Geԭ�Ӱ뾶����Si��Si��O����С��Ge��O������SiO2���ܸ����۵���ߣ�
��3��Bԭ���������3�����ӣ���3��-OH�γ�3�����ۼ������Ϊsp2�ӻ�����ˮ�ƻ������ᾧ���е�������������������ˮ�γɷ��Ӽ������ʹ�ܽ������
�ʴ�Ϊ��sp2����ˮ�ƻ������ᾧ���е�������������������ˮ�γɷ��Ӽ������ʹ�ܽ������
��4��ԭ�ӷ�������Ϊ��0.5��0.2��0.5����Csԭ��λ�ھ������ڣ�ԭ�ӷ�������Ϊ��0��0.3��0.5������1.0��0.3��0.5����Csԭ��λ�ھ�����yz���ϣ�ԭ�ӷ�������Ϊ��0.5��0.8��1.0������0.5��0.8��0����Csԭ��λ�ھ���xy���ϣ�ԭ�ӷ�������Ϊ��0��0.7��1.0������1.0��0.7��1.0����0��0.7��0����Csԭ��λ�ھ���ƽ����y������ϣ����þ�̯���ɼ���þ����й���Csԭ��4�������뾧���ܶ����㹫ʽ�ɵã�![]() ���ʴ�Ϊ4��
���ʴ�Ϊ4��![]() ��
��

����Ŀ������Ҫ��������и�С�⣺
��.��1��������ϡ��ǿ�ᡢǿ�Ӧ����1mol H2O��l��ʱ�ų�57.3kJ��������д����ʾϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ________ ��
��2����֪���ұ��������Ʊ���ϩ��Ӧ�����ڼ������ʱ���˴��ɿ���Ϊ![]() ��
�� ![]()
![]()
![]() +H2��g��
+H2��g��
��ѧ�� | C��H | C��C | C=C | H��H |
����/kJ��mol��1 | 412 | 348 | 612 | 436 |
����������Ӧ����H��________ kJ��mol��1��
��.25 ��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ����ش��������⣺
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.7��10��5 | K1��4.3��10��7 K2��5.6��10��11 | 3.0��10��8 |
��1��CH3COOH��H2CO3��HClO��������ǿ������˳��Ϊ_______��
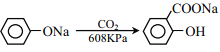
��2��������CO2����ͨ��NaClO��Һ�У�д����Ӧ�����ӷ���ʽ��________��