��Ŀ����
����ѧƽ���ƶ�ԭ����ͬ��Ҳ����������ƽ��
��1����֪�ڰ�ˮ�д�������ƽ�⣺NH3��H2O NH3��H2O
NH3��H2O  NH��OH��
NH��OH��
��ˮ�м���MgCl2����ʱ��ƽ������ �ƶ���OH����Ũ��
��Ũ��ˮ�м�������NaOH���壬ƽ���� �ƶ�����ʱ�����������ǣߣߣߣߣߣߣߣߣߣߣߡ�
��2���Ȼ���ˮ������ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ����Ȼ�����Һ�м���̼��Ʒ�ĩ������̼������ܽ⣬��������ɫ���壬�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�ͬʱ�к��ɫ�������ɣ���ԭ����
��3����Mg(OH)2������Һ�м���NH4Cl��Һ������ ��ԭ
��Ϊ
����ij��Ԫ�� H2A �ĵ��뷽��ʽ�ǣ�H2A=H++HA��HA- A2-+H+���ش��������⣺
A2-+H+���ش��������⣺
��1��H2A�� ���ǿ����ʡ���������ʡ��ǵ���ʡ���
��2��Na2A ��Һ�� ������ԡ��������ԡ������ԡ����������ǣ������ӷ���ʽ��ʾ�� ��
��3��NaHA ��Һ�� ������ԡ��������ԡ������ԡ����������ǣ������ӷ���ʽ��ʾ�� ��
��4���� 0��1mol��L-1NaHA ��Һ�� pH=2���� 0��1mol��L-1 H2A��Һ�������ӵ����ʵ���Ũ�ȿ��� 0.11mol��L����������������������������ǣ� ��
��5��0��1mol��L NaHA��Һ�и�����Ũ���ɴ�С��˳���� ��
��1����֪�ڰ�ˮ�д�������ƽ�⣺NH3��H2O
 NH3��H2O
NH3��H2O  NH��OH��
NH��OH����ˮ�м���MgCl2����ʱ��ƽ������ �ƶ���OH����Ũ��
��Ũ��ˮ�м�������NaOH���壬ƽ���� �ƶ�����ʱ�����������ǣߣߣߣߣߣߣߣߣߣߣߡ�
��2���Ȼ���ˮ������ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ����Ȼ�����Һ�м���̼��Ʒ�ĩ������̼������ܽ⣬��������ɫ���壬�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�ͬʱ�к��ɫ�������ɣ���ԭ����
��3����Mg(OH)2������Һ�м���NH4Cl��Һ������ ��ԭ
��Ϊ
����ij��Ԫ�� H2A �ĵ��뷽��ʽ�ǣ�H2A=H++HA��HA-
 A2-+H+���ش��������⣺
A2-+H+���ش��������⣺��1��H2A�� ���ǿ����ʡ���������ʡ��ǵ���ʡ���
��2��Na2A ��Һ�� ������ԡ��������ԡ������ԡ����������ǣ������ӷ���ʽ��ʾ�� ��
��3��NaHA ��Һ�� ������ԡ��������ԡ������ԡ����������ǣ������ӷ���ʽ��ʾ�� ��
��4���� 0��1mol��L-1NaHA ��Һ�� pH=2���� 0��1mol��L-1 H2A��Һ�������ӵ����ʵ���Ũ�ȿ��� 0.11mol��L����������������������������ǣ� ��
��5��0��1mol��L NaHA��Һ�и�����Ũ���ɴ�С��˳���� ��
����1���� ��С �� �д̼����������
��2��Fe3+ + 3H2O Fe(OH)3 + 3H+ �� CaCO3 + 2H��=== Ca2+ + H2O +CO2��
Fe(OH)3 + 3H+ �� CaCO3 + 2H��=== Ca2+ + H2O +CO2��
̼�������H+ ,�ٽ��Ȼ�����ˮ�⣬ʹˮ�����Fe(OH)3��������,�γɺ��ɫ����
��3����Һ���ܽ⡡��Һ�д���ƽ�� Mg(OH)2 (S) Mg2��(aq)+2OH��(aq) ,����NH4Cl��Һ�ᷢ��OH-+NH4+=NH3��H2O ������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�
Mg2��(aq)+2OH��(aq) ,����NH4Cl��Һ�ᷢ��OH-+NH4+=NH3��H2O ������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�
����1��ǿ�����
��2������ A2-+H2O HA-+OH-
HA-+OH-
(3)���� HA�� H++ A2��
H++ A2��
��4���� ��H2A��1�����������H+����HA-�ĵ���
��5��c(Na��)��c(HA��)>c(H��)>c(A2��)>c(OH��)
��2��Fe3+ + 3H2O
 Fe(OH)3 + 3H+ �� CaCO3 + 2H��=== Ca2+ + H2O +CO2��
Fe(OH)3 + 3H+ �� CaCO3 + 2H��=== Ca2+ + H2O +CO2�� ̼�������H+ ,�ٽ��Ȼ�����ˮ�⣬ʹˮ�����Fe(OH)3��������,�γɺ��ɫ����
��3����Һ���ܽ⡡��Һ�д���ƽ�� Mg(OH)2 (S)
 Mg2��(aq)+2OH��(aq) ,����NH4Cl��Һ�ᷢ��OH-+NH4+=NH3��H2O ������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�
Mg2��(aq)+2OH��(aq) ,����NH4Cl��Һ�ᷢ��OH-+NH4+=NH3��H2O ������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�����1��ǿ�����
��2������ A2-+H2O
 HA-+OH-
HA-+OH- (3)���� HA��
 H++ A2��
H++ A2����4���� ��H2A��1�����������H+����HA-�ĵ���
��5��c(Na��)��c(HA��)>c(H��)>c(A2��)>c(OH��)
��1������ƽ��NH3��H2O NH3��H2O
NH3��H2O  NH��OH����������ˮ�м���MgCl2����ʱ��Mg2����2OH��=Mg(OH)2����OH��Ũ�ȼ�С��ƽ���������ƶ�����Ũ��ˮ�м�������NaOH����ʱ��OH��Ũ������ƽ�������ƶ���ͬʱ�����ܽ�����зų��������ȣ�NH3��H2O
NH��OH����������ˮ�м���MgCl2����ʱ��Mg2����2OH��=Mg(OH)2����OH��Ũ�ȼ�С��ƽ���������ƶ�����Ũ��ˮ�м�������NaOH����ʱ��OH��Ũ������ƽ�������ƶ���ͬʱ�����ܽ�����зų��������ȣ�NH3��H2O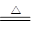 NH3����H2O�����д̼��������ݳ�
NH3����H2O�����д̼��������ݳ�
��2���Ȼ���ˮ������ӷ���ʽΪFe3+ + 3H2O Fe(OH)3 + 3H+�������Ȼ�����Һ�м���̼��Ʒ�ĩ��CaCO3 + 2H��=== Ca2+ + H2O +CO2����H+Ũ�ȼ�С��ƽ���������ƶ���ʹˮ�����Fe(OH)3�������ɣ����к��ɫFe(OH)3��������
Fe(OH)3 + 3H+�������Ȼ�����Һ�м���̼��Ʒ�ĩ��CaCO3 + 2H��=== Ca2+ + H2O +CO2����H+Ũ�ȼ�С��ƽ���������ƶ���ʹˮ�����Fe(OH)3�������ɣ����к��ɫFe(OH)3��������
��3����Һ�д����ܽ�ƽ�⣺Mg(OH)2 (S) Mg2��(aq)+2OH��(aq) ,����NH4Cl��Һ�ᷢ��OH-+NH4+=NH3��H2O ������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�
Mg2��(aq)+2OH��(aq) ,����NH4Cl��Һ�ᷢ��OH-+NH4+=NH3��H2O ������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�
����1��H2Aȫ������Ϊ���ӣ���Ϊǿ�����
��2������HA-����Һ�д���ƽ�⣺HA- A2-+H+����Na2A�������A2-��ˮ��ʹ��Һ�ʼ��ԣ�A2-+H2O
A2-+H+����Na2A�������A2-��ˮ��ʹ��Һ�ʼ��ԣ�A2-+H2O HA-+OH-
HA-+OH-
��3��NaHA�������HA����ǿ��H2A��Ӧ�����ӣ���ˮ�⣬���ɵ���ʹ��Һ�����ԣ�HA�� H++ A2������NaHA��ҺΪ����
H++ A2������NaHA��ҺΪ����
��4��0��1mol��L-1NaHA ��Һ��c(H+)=0��01mol��L-1��0��1mol��L-1 H2A��Һ��ȫ�����룺H2A=H++HA������������c(H+)��c(HA��)Ϊ0��1mol��L-1������H2A��1�����������H+����HA-�ĵ��룬��c(HA��)���ӳ���c(H+)�ض�С��0��01mol��L-1����Һ��c(H+)�ض�С��0��11mol��L-1
��5��0��1mol��L NaHA��Һ����Ҫ���ڵ�����Ϊc(Na��)��c(HA��)������c(HA��)���ֵ��룬c(Na��)��c(HA��)����Һ�����ԣ�ͬʱ����H2O H����OH������c(H��)>c(A2��)>c(OH��)���� NaHA��Һ�и�����Ũ���ɴ�С��˳����c(Na��)��c(HA��)>c(H��)>c(A2��)>c(OH��)
H����OH������c(H��)>c(A2��)>c(OH��)���� NaHA��Һ�и�����Ũ���ɴ�С��˳����c(Na��)��c(HA��)>c(H��)>c(A2��)>c(OH��)
 NH3��H2O
NH3��H2O  NH��OH����������ˮ�м���MgCl2����ʱ��Mg2����2OH��=Mg(OH)2����OH��Ũ�ȼ�С��ƽ���������ƶ�����Ũ��ˮ�м�������NaOH����ʱ��OH��Ũ������ƽ�������ƶ���ͬʱ�����ܽ�����зų��������ȣ�NH3��H2O
NH��OH����������ˮ�м���MgCl2����ʱ��Mg2����2OH��=Mg(OH)2����OH��Ũ�ȼ�С��ƽ���������ƶ�����Ũ��ˮ�м�������NaOH����ʱ��OH��Ũ������ƽ�������ƶ���ͬʱ�����ܽ�����зų��������ȣ�NH3��H2O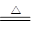 NH3����H2O�����д̼��������ݳ�
NH3����H2O�����д̼��������ݳ���2���Ȼ���ˮ������ӷ���ʽΪFe3+ + 3H2O
 Fe(OH)3 + 3H+�������Ȼ�����Һ�м���̼��Ʒ�ĩ��CaCO3 + 2H��=== Ca2+ + H2O +CO2����H+Ũ�ȼ�С��ƽ���������ƶ���ʹˮ�����Fe(OH)3�������ɣ����к��ɫFe(OH)3��������
Fe(OH)3 + 3H+�������Ȼ�����Һ�м���̼��Ʒ�ĩ��CaCO3 + 2H��=== Ca2+ + H2O +CO2����H+Ũ�ȼ�С��ƽ���������ƶ���ʹˮ�����Fe(OH)3�������ɣ����к��ɫFe(OH)3����������3����Һ�д����ܽ�ƽ�⣺Mg(OH)2 (S)
 Mg2��(aq)+2OH��(aq) ,����NH4Cl��Һ�ᷢ��OH-+NH4+=NH3��H2O ������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�
Mg2��(aq)+2OH��(aq) ,����NH4Cl��Һ�ᷢ��OH-+NH4+=NH3��H2O ������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�����1��H2Aȫ������Ϊ���ӣ���Ϊǿ�����
��2������HA-����Һ�д���ƽ�⣺HA-
 A2-+H+����Na2A�������A2-��ˮ��ʹ��Һ�ʼ��ԣ�A2-+H2O
A2-+H+����Na2A�������A2-��ˮ��ʹ��Һ�ʼ��ԣ�A2-+H2O HA-+OH-
HA-+OH-��3��NaHA�������HA����ǿ��H2A��Ӧ�����ӣ���ˮ�⣬���ɵ���ʹ��Һ�����ԣ�HA��
 H++ A2������NaHA��ҺΪ����
H++ A2������NaHA��ҺΪ������4��0��1mol��L-1NaHA ��Һ��c(H+)=0��01mol��L-1��0��1mol��L-1 H2A��Һ��ȫ�����룺H2A=H++HA������������c(H+)��c(HA��)Ϊ0��1mol��L-1������H2A��1�����������H+����HA-�ĵ��룬��c(HA��)���ӳ���c(H+)�ض�С��0��01mol��L-1����Һ��c(H+)�ض�С��0��11mol��L-1
��5��0��1mol��L NaHA��Һ����Ҫ���ڵ�����Ϊc(Na��)��c(HA��)������c(HA��)���ֵ��룬c(Na��)��c(HA��)����Һ�����ԣ�ͬʱ����H2O
 H����OH������c(H��)>c(A2��)>c(OH��)���� NaHA��Һ�и�����Ũ���ɴ�С��˳����c(Na��)��c(HA��)>c(H��)>c(A2��)>c(OH��)
H����OH������c(H��)>c(A2��)>c(OH��)���� NaHA��Һ�и�����Ũ���ɴ�С��˳����c(Na��)��c(HA��)>c(H��)>c(A2��)>c(OH��)
��ϰ��ϵ�д�
�����Ŀ

 ���ɾ綾��HCN������������⣺
���ɾ綾��HCN������������⣺ 0.2mol����_ __��_ __�������ӵ����ʵ���
0.2mol����_ __��_ __�������ӵ����ʵ��� HCN��OH��
HCN��OH��