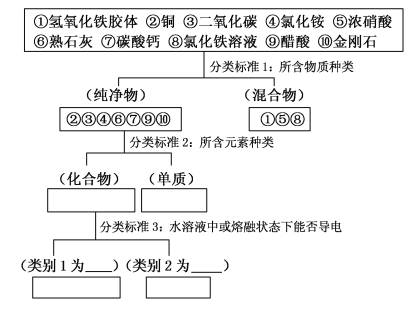
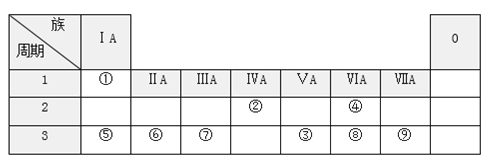
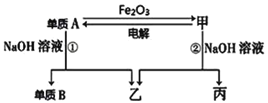
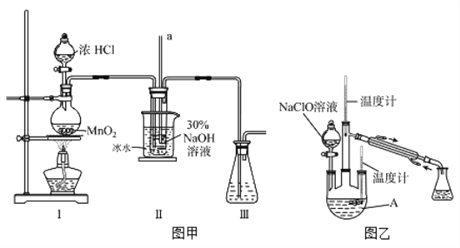
��Ŀ����
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ,�á�84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش���������:
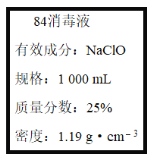
��1��ijͬѧȡ100 mL�á�84����Һ��,ϡ�ͺ���������,ϡ�ͺ����Һ��c(Na+)��____mol��L-1��
��2����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ������˵����ȷ����____(����ĸ)��
a.��ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ�������
b.����ƿ������ˮϴ����Ӧ��ɺ����������Һ����
c.���ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
d.��Ҫ����NaClO���������Ϊ143.0 g
���𰸡� 0.04 c
����������1������c=1000�Ѧ�/M������Һ�����ʵ���Ũ�ȣ�����ϡ���������ʵ���������㣻
��2��a����������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ������
b������ƿ���ܺ�ɣ�
c�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС��
d������m��cVM������Ҫ���ʵ�������
��1���á�84����Һ�������ʵ���Ũ��c��1000��1.19��25%/74.5 mol��L��1��4.0mol/L��ϡ���������ʵ����ʵ������䣬��ϡ�ͺ�������Ƶ����ʵ���Ũ����4.0mol/L��100��0.04mol/L������ϡ�ͺ����Һ��c(Na+)��0.04 mol��L-1��
��2��a������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��������ȡ�����ܽ⣨ϡ�ͣ�����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������������ƽ����Ͳ����ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����Ҫ���ǣ�Բ����ƿ�ͷ�Һ©��������Ҫ����������ͷ�ιܣ�a����
b������ƿ������ˮϴ������������Һ���ƣ�����ƿ���ܺ�ɣ�b����
c�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС������c��n/V��֪��ҺŨ��ƫ�ͣ�c��ȷ��
d������480mL��NaClO��������Ϊ25%������Һ�������õ�����ƿ�������ʵ���Ũ��Ϊ4.0mol/L����Ҫѡ��500mL����ƿ��������Ҫ���ʵ�����m=4.0mol/L��74.5g/mol��0.5L=149g��d����
��ѡc��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��800��ʱ��2L�ܱ������ڷ�Ӧ��2NO��g��+O2��g��![]() 2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��1����ƽ��ʱNO��ת����_________________��
��2����ͼ�б�ʾNO2�ı仯��������________����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v��O2��=____________��

��3����˵���÷�Ӧһ���ﵽƽ��״̬����_____________��
a��v��NO2��=2v��O2�� b����������ɫ���ֲ���
c��2v����NO��=v����O2�� d��������ѹǿ���ֲ���
��4��������÷�Ӧ�ķ�Ӧ���ʵ���___________________��
a����ʱ�����NO2���� b���ʵ������¶�
c������O2��Ũ�� d��ѡ���Ч����