��Ŀ����
����Ŀ�������仯�������ճ���������;�ȽϹ㷺��
(1)��������(Na2FeO4)��һ�����͵���ɫ��������������ز��ϡ���Fe(NO3)3��NaClO��Ϻ��ڼ��������·�����Ӧ���Ƶø������ƣ��÷�Ӧ�����ӷ���ʽΪ________________________��
(2)����������(Fe3O4)���������ϡ���������ϡ������͵��Ӳ��ϵȡ�����������Ŀǰ�Ʊ�����Fe3O4����Ҫ����֮һ����������ͼ��ʾ��

��Ϊ�õ��ϴ���������Fe3O4��FeSO4��7H2O��FeCl3��6H2O�����ʵ���֮�����Ϊ________����ʵ�ʲ���ʱ��ȴ���ѿ�����һ������ԭ����_____________________________��
�������Ͷ�ϱ������£�����Fe3O4�Ƿ������ȫ��ʵ�������_________________________��
(3)�̷�(FeSO4��7H2O)������ȱ����ƶѪҩƷ����Ҫ�ɷ֡��ⶨ�̷���Ʒ��FeSO4��7H2O�����ķ������£�
a����ȡ3.0 g�̷���Ʒ�����Ƴ�250.00 mL��Һ��
b����ȡ25.00 mL a����Һ����ƿ�У�
c����0.010 00 mol��L��1����KMnO4��Һ�ζ����յ㣬����KMnO4��Һ��ƽ�����Ϊ20.00 mL���ζ�ʱ������Ӧ�����ӷ���ʽΪ5Fe2����MnO![]() ��8H��===5Fe3����Mn2����4H2O��
��8H��===5Fe3����Mn2����4H2O��
��0.010 00 mol��L��1KMnO4��ҺӦ������ͼ��ʾ����________(��ס����ҡ�)�У��ζ��յ��������__________________________________��
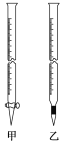
�ڲ�Ʒ��FeSO4��7H2O����������Ϊ________(С�������1λ����)��
���������������ⶨ����Ʒ��FeSO4��7H2O����������ƫ��(�ⶨ�����в��������ɺ���)�����ܵ�ԭ����___________________________��
���𰸡�
��������(1)������֪����Ӧ��ΪFe3����ClO����OH������������FeO![]() ��ClO������ԭӦ����Cl�������ݵ�ʧ�����غ㡢����غ��ԭ���غ����ƽ�����ӷ���ʽ��(2)��Fe3O4��Fe2����Fe3���ĸ�����Ϊ1��2����ԭ����FeSO4��7H2O��FeCl3��6H2O�����ʵ���֮��Ϊ1��2��Fe2���н�ǿ�Ļ�ԭ�ԣ��ڿ������ױ�������Ӱ��Ͷ�ϱȡ������Ͷ�ϱ�ʱ��������ȫ������Һ�в�����Fe2����Fe3�����ʿ���KSCN��Һ���顣(3)��KMnO4��Һ����ǿ�����ԣ��ܸ�ʴ���ʱ���������ʽ�ζ����С��ζ����յ�ʱ����������һ��KMnO4��Һ���ٷ�Ӧ��ʹ��Һ��dz��ɫ�����ɷ�Ӧ�����ӷ���ʽ��֪��n(Fe2��)��5n(MnO
��ClO������ԭӦ����Cl�������ݵ�ʧ�����غ㡢����غ��ԭ���غ����ƽ�����ӷ���ʽ��(2)��Fe3O4��Fe2����Fe3���ĸ�����Ϊ1��2����ԭ����FeSO4��7H2O��FeCl3��6H2O�����ʵ���֮��Ϊ1��2��Fe2���н�ǿ�Ļ�ԭ�ԣ��ڿ������ױ�������Ӱ��Ͷ�ϱȡ������Ͷ�ϱ�ʱ��������ȫ������Һ�в�����Fe2����Fe3�����ʿ���KSCN��Һ���顣(3)��KMnO4��Һ����ǿ�����ԣ��ܸ�ʴ���ʱ���������ʽ�ζ����С��ζ����յ�ʱ����������һ��KMnO4��Һ���ٷ�Ӧ��ʹ��Һ��dz��ɫ�����ɷ�Ӧ�����ӷ���ʽ��֪��n(Fe2��)��5n(MnO![]() )��5��0.010 00 mol��L��1��0.020 0 L��250.00 mL��25.00 mL��0.01 mol����w(FeSO4��7H2O)��0.01 mol��278 g��mol��1��3.0 g��100%��92.7%��������������ƫ�ͣ�˵����Һ�������ʻ�Fe2���ѱ�������
)��5��0.010 00 mol��L��1��0.020 0 L��250.00 mL��25.00 mL��0.01 mol����w(FeSO4��7H2O)��0.01 mol��278 g��mol��1��3.0 g��100%��92.7%��������������ƫ�ͣ�˵����Һ�������ʻ�Fe2���ѱ�������

����Ŀ��һ���¶��£�������Ӧ��FeO��s��+CO��g��![]() Fe��s��+CO2��g�� ��H����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����±���
Fe��s��+CO2��g�� ��H����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����±���
�¶�/�� | 1000 | 1100 |
ƽ�ⳣ�� | 0.68 | 0.50 |
��ش��������⣺
��1���÷�Ӧ�Ħ�H 0
��2��T��ʱ����FeO��s����CO��g����3.0mol����10L���ܱ������У���Ӧ�ﵽƽ����COת����Ϊ��1��c��CO2����0.15molL��1�����¶�T ������ڡ����ڡ�������1000������ʱ�����������������ٳ���2.0 mol CO��g�����ٴ�ƽ��ʱ���COת����2�����1 ��2�������������
