��Ŀ����
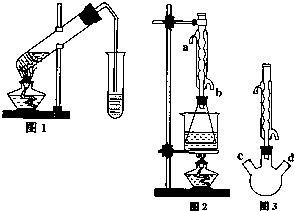 ������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᣮijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����
������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᣮijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����| �������� | �Ҵ� | ���� | |
| �е� | 77.1�� | 78.5�� | 117.9�� |
��I��ȷ����20.0g����������Ʒ����ƿ�У���0.50mol?L-1NaOH�ζ�����̪��ָʾ�������յ�ʱ����NaOH��Һ�����Ϊ40.0mL
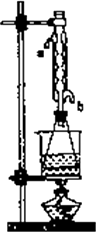
��II����ȡ20.0g���������ֲ�Ʒ��250mL��ƿ�У�����100mL 2.1mol?L-1NaOH��Һ��Ͼ��Ⱥ�װ�������䣬��ˮԡ�ϼ��Ȼ���Լ1Сʱ��װ����ͼ2��ʾ������ȴ����0.50mol?L-1HCl�ζ�������NaOH���յ�ʱ������������Ϊ20.0mL��
�ش��������⣺
��1��ʵ�飨II������ˮ����ˮ���ܵ�
��2������ʵ�飨I������II�����������ݼ���ֲ�����������������������Ϊ
��3��ʵ�������ͬѧ�ǶԴֲ�Ʒ�����������ĺ������߽������ۣ�
��������Ϊ��ʵ�飨II�������������齫ͼ2�е���ƿ��Ϊ����ƿ��װ����ͼ3��������ƿ��c��d��װ����ص�����������ǡ���IJ�����������߲ⶨ�ľ�ȷ�ȣ�����Ϊ������ƿ��c��d��װ����ص�����������ǣ�
A��װ���¶ȼƣ��ϸ���Ʒ�Ӧ�¶�
B��ʵ���о�����ƿ�ڣ��ò������н���
C���ڷ�Ӧ���ڣ������Ȱ�װ�ķ�Һ©������һ������NaOH��Һ
�ڻ���ͬѧ��Ϊ�Ľ�������������ȡװ�ã�ͼ1��������߲��ʣ�������һ���Ľ�����
��������1��������������������ͨˮЧ������ɣ�
��2����������ʵ�飨I������II�����������ݼ���Ӧԭ��������ֲ�������������������������
��3�����ɱ������������ķе�����������¶ȹ��ߣ����ܵ���������������������ʧ��
�ڽ��Թܸij�Բ����ƿ��Ȼ���װ�¶ȼƿ����¶ȣ�������������IJ��ʣ�
��2����������ʵ�飨I������II�����������ݼ���Ӧԭ��������ֲ�������������������������
��3�����ɱ������������ķе�����������¶ȹ��ߣ����ܵ���������������������ʧ��
�ڽ��Թܸij�Բ����ƿ��Ȼ���װ�¶ȼƿ����¶ȣ�������������IJ��ʣ�
�����1����������������ͨˮ��ʹˮ�������������ֽӴ�������Ч���ã��ʴ�bͨˮ��
�ʴ�Ϊ��b��
��2���ɣ���֪�����������Ʒ�Ӧ�������������е��������ᣬ����20g����������Ʒ�к�����������ʵ���Ϊ��0.50mol?L-1��0.04L=0.02mol��
�У���֪����������ˮ�����ɵ�����������������ĵ��������Ƶ����ʵ���Ϊ��2.1mol?L-1��0.1L-0.50mol?L-1��0.02L=0.20mol��
��������ˮ�����ɵ���������ʵ���Ϊ��0.20mol-0.02mol=0.18mol��
�������������ʵ���������������ʵ���������20g����������Ʒ�к�����������������Ϊ��88g/mol��0.18g=15.84g��
��������������������
��100%=79.2%��
�ʴ�Ϊ��79.2%��
��3����A��װ���¶ȼƣ��ܹ��ϸ���Ʒ�Ӧ�¶ȣ����������������������ʧ���ܹ���С��Ӧ����A��ȷ��
B��ʵ���о�����ƿ�ڣ��ò������н��裬�ᵼ����������������ӻ��Һ�лӷ�������Ӱ��ⶨ�������B����
C���ڷ�Ӧ���ڣ������Ȱ�װ�ķ�Һ©������һ������NaOH��Һ��������������Ũ�ȣ���֤����������ȫ��Ӧ�����Լ�С�ⶨ����C��ȷ��
��ѡAC��
����������������ȡ�����У��Ҵ��������ܹ��ӷ�������Ӱ�������������IJ��ʣ�������Բ����ƿ����Թܲ���װ�¶ȼƿ��Ʒ�Ӧ�¶ȵķ�����ͨ�����������IJ��ʣ�
�ʴ�Ϊ����Բ����ƿ����Թܲ���װ�¶ȼƿ��Ʒ�Ӧ�¶ȣ�
�ʴ�Ϊ��b��
��2���ɣ���֪�����������Ʒ�Ӧ�������������е��������ᣬ����20g����������Ʒ�к�����������ʵ���Ϊ��0.50mol?L-1��0.04L=0.02mol��
�У���֪����������ˮ�����ɵ�����������������ĵ��������Ƶ����ʵ���Ϊ��2.1mol?L-1��0.1L-0.50mol?L-1��0.02L=0.20mol��
��������ˮ�����ɵ���������ʵ���Ϊ��0.20mol-0.02mol=0.18mol��
�������������ʵ���������������ʵ���������20g����������Ʒ�к�����������������Ϊ��88g/mol��0.18g=15.84g��
��������������������
| 15.84g |
| 20g |
�ʴ�Ϊ��79.2%��
��3����A��װ���¶ȼƣ��ܹ��ϸ���Ʒ�Ӧ�¶ȣ����������������������ʧ���ܹ���С��Ӧ����A��ȷ��
B��ʵ���о�����ƿ�ڣ��ò������н��裬�ᵼ����������������ӻ��Һ�лӷ�������Ӱ��ⶨ�������B����
C���ڷ�Ӧ���ڣ������Ȱ�װ�ķ�Һ©������һ������NaOH��Һ��������������Ũ�ȣ���֤����������ȫ��Ӧ�����Լ�С�ⶨ����C��ȷ��
��ѡAC��
����������������ȡ�����У��Ҵ��������ܹ��ӷ�������Ӱ�������������IJ��ʣ�������Բ����ƿ����Թܲ���װ�¶ȼƿ��Ʒ�Ӧ�¶ȵķ�����ͨ�����������IJ��ʣ�
�ʴ�Ϊ����Բ����ƿ����Թܲ���װ�¶ȼƿ��Ʒ�Ӧ�¶ȣ�
���������⿼����������������ȡ����Ŀ�Ѷ��еȣ�ע���������������ķ�Ӧԭ����װ��ѡ��������ע�ض�ѧ������֪ʶѵ���ͼ����ͬʱ�����ض�ѧ��ʵ����������������ͷ����뼼�ɵ�ָ����ѵ�������������ѧ����ʵ�����������Ӧ������������ѧ����ѧ��������

��ϰ��ϵ�д�
�����Ŀ
 ������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH
������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH 



 (08�Ϻ��ɽ���ģ��)������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ�����������ͼ
(08�Ϻ��ɽ���ģ��)������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ�����������ͼ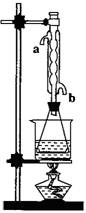 �Ƕ�������������������װ�ã�Ϊ����߲��ʣ������
�Ƕ�������������������װ�ã�Ϊ����߲��ʣ������